Dyslipidemia remains among the main causes of cardiovascular disease (CVD), leading to more than 21,000 deaths per year in Switzerland. Reducing low-density lipoprotein cholesterol (LDL-C) levels is a cornerstone of CVD management in the 2021 update of the European Society of Cardiology (ESC) Guidelines on cardiovascular disease prevention in clinical practice.
Dyslipidemia remains among the main causes of cardiovascular disease (CVD), leading to more than 21,000 deaths per year in Switzerland [1]. Reducing low-density lipoprotein cholesterol (LDL-C) levels is a cornerstone of CVD management in the 2021 update of the European Society of Cardiology (ESC) Guidelines on cardiovascular disease prevention in clinical practice [2]. In Switzerland, up to a third of the population has an unfavorable lipid profile [3].
LDL-C: the lower the better
The causal relationship between increased LDL-C values and atherosclerotic cardiovascular disease (ASCVD) has been consistently demonstrated in a long-standing track record of genetic, epidemiologic, and randomized studies [4,5]. Further, randomized clinical trials (RCTs) and meta-analyses have highlighted that pharmacological reduction of LDL-C decreases the risk of ASCVD proportionally to the absolute LDL-C reduction, with no lower LDL-C limit at which benefits diminish and harm occurs [6-9]. In response to these insights, the 2019 update of the ESC and the European Atherosclerosis Society (EAS) Guidelines for the management of dyslipidemias defines even stricter LDL-C goals as compared to the 2016 version, for individuals at moderate, high, and very high CVD risk [4]. The Lipids and Atherosclerosis Working Group (AGLA) of the Swiss Society of Cardiology (SGK) has followed these stringent LDL-C goals in the 2020 update of their guidelines for prevention of atherosclerosis [10].
New ESC Guidelines on cardiovascular disease prevention in clinical practice
The ESC Guidelines on cardiovascular disease prevention in clinical practice provide practical guidance on treatment of the major causal and modifiable ASCVD risk factors, such as smoking, LDL-C, high blood pressure (BP), diabetes mellitus (DM), and adiposity [2]. In the 2021 update of these guidelines, a stepwise, individualized approach is suggested: cessation of smoking, lifestyle optimization and maintaining a systolic blood pressure (SBP) below 160 mmHg lay foundation to CVD prevention and apply to all individuals, regardless of age and disease status. Based on individual CVD risk pharmacological risk factor treatment (STEP 1), and treatment intensification (STEP 2) should then be implemented, with consideration of risk modifiers, lifetime CVD risk, treatment benefit and patient preferences. Specific goals for LDL-C, BP, and glycemic control in patients with DM remain as recommended in the corresponding ESC Guidelines [4,11,12].
Risk classification and stratification are key elements of CV care in primary prevention: In primary prevention, the decision to initiate risk factor modification is largely based on each patient’s estimated CVD risk. Patients with documented ASCVD, chronic kidney disease (CKD), familial hypercholesterolemia (FH) or long-standing DM are at least at high CVD risk [4]. Yet, CV events are rarely the result of a single risk factor but rather the consequence of several combined risk factors. To facilitate and guide clinical management decisions and avoid both under- and overtreatment, a multitude of CVD risk estimation systems has been developed in the past decades. The Framingham system, which was validated against US cohorts, has pioneered the field of CVD risk estimation in 1998 [13]. Since then, several other risk assessment instruments have been developed, which include more recently described risk factors and/or have been validated with cohort data from other countries. Examples include the American College of Cardiology/American Heart Association (ACC/AHA) pooled cohort equations, the Scottish ASSIGN score, the QRISK prediction algorithm, which was calibrated based on UK cohorts, and the Systematic Coronary Risk Evaluation (SCORE) system, which was validated against a data set of 12 European cohort studies [14–16]. In Switzerland, for apparently healthy individuals without ASVD, FH, CKD, or DM, the AGLA risk score estimated the individual 10-year risk of a fatal coronary event or a non-fatal myocardial event [10].
If country-specific risk charts were not available, the ESC has until recently recommended to estimate CVD risk of apparently healthy individuals with the SCORE model, based on two European risk regions, with either high or low CVD risk [4]. With the recent update of the ESC Guidelines on cardiovascular disease prevention in clinical practice, the new SCORE2 and SCORE2-OP algorithms have replaced the older SCORE risk estimation chart.
SCORE2 replaces SCORE for apparently healthy individuals aged 40–69: The now obsolete SCORE algorithm only included fatal CVD outcomes, thus underestimating total CVD burden, which has shifted in the recent decades towards non-fatal outcomes, particularly in younger individuals [17]. Furthermore, with only two risk regions, SCORE did not allow for substantial differentiation of country-specific risk. Finally, SCORE was calibrated with CVD rates of population cohorts from the 1980s.
The novel SCORE2 risk model incorporates contemporary age-, sex-, and region-specific CVD risk factor values and CV incidence rates across Europe (Fig. 1) [17]. In contrast to SCORE, SCORE2 provides risk estimates for the combined outcome of fatal and nonfatal CVD events such as myocardial infarction and stroke, thus better estimating CVD total risk, especially in younger individuals, where case-fatality rates are lower [17]. European countries are now grouped into four risk regions, with low, intermediate, high, and very high risk, according to their most recent World Health Organization (WHO) age- and sex-standardized overall CVD mortality rates, thus improving the two-level regional stratification of SCORE. As in SCORE, Switzerland remains a low-risk country in SCORE2. Moreover, SCORE2 accounts for the impact of competing risks by non-CVD deaths, thereby preventing over-estimation of CVD risk and treatment benefit in individuals with high risk of non-CVD deaths [17].
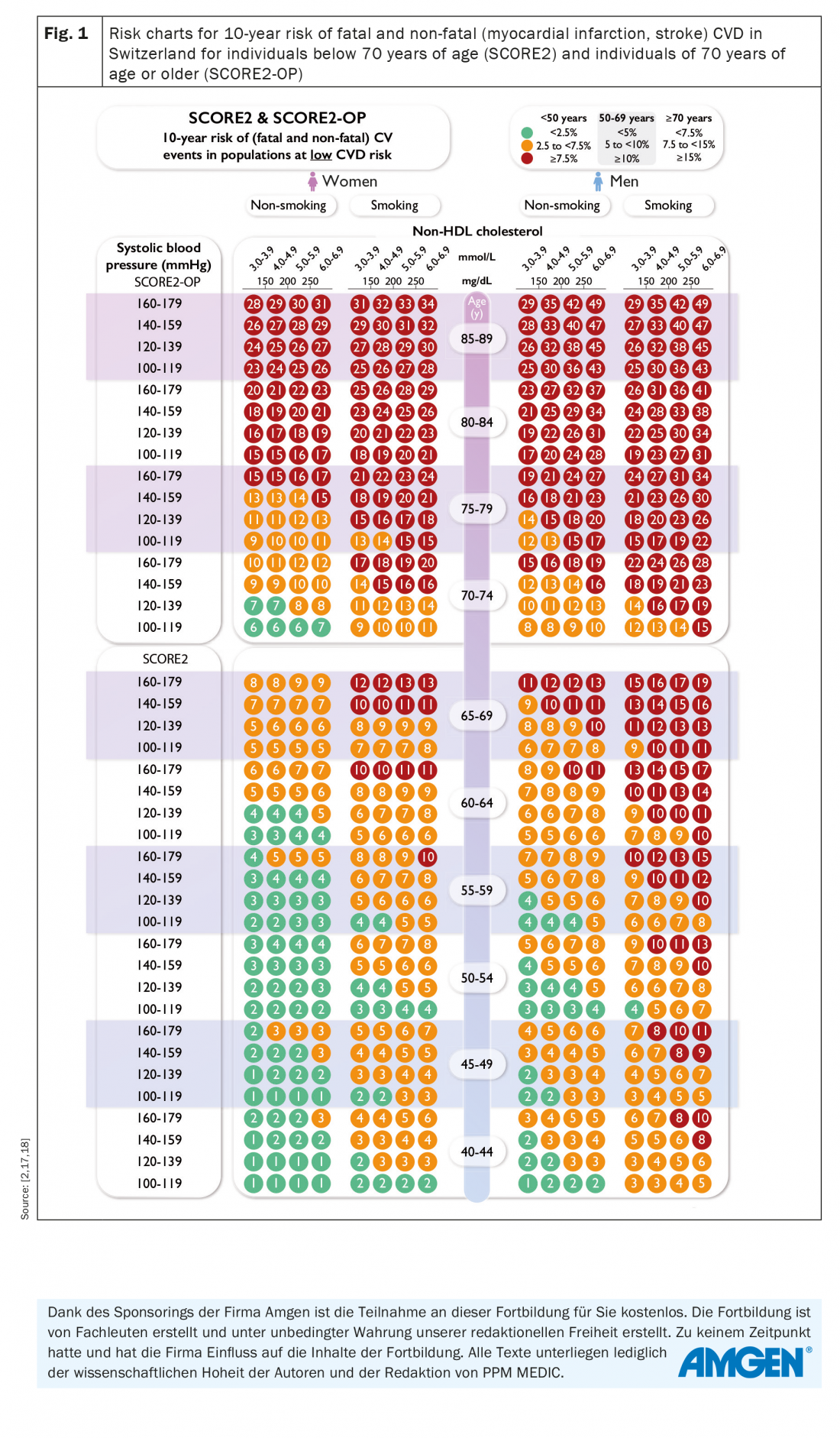
SCORE2-OP (Older Persons) – optimized for individuals ≥70 years of age: The impact of non-competing risks by non-CVD mortality increases with age, leading to a progressive dissociation of CVD-free survival from overall survival. Traditional risk models do not consider competing risks of non-CVD mortality, thus overestimating the actual 10-year CVD risk and hence the potential benefit of risk factor treatment. For apparently healthy people aged 70 years or older, the SCORE2-OP algorithm estimates the 10-year fatal and non-fatal CVD events adjusted for competing risks (Fig. 1) [18].
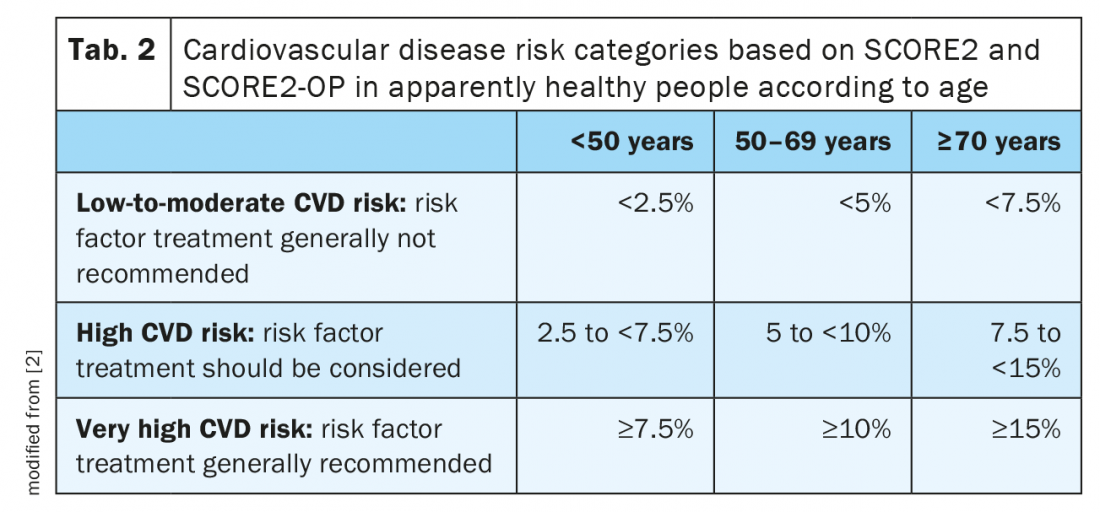
Age-specific risk thresholds for treatment initiation: A key element of the updated ESC CVD prevention guidelines is the introduction of age-dependent risk level thresholds to avoid undertreatment in the young and overtreatment in the elderly (Tab. 2). Furthermore, it is suggested that risk categories should not automatically be translated into treatment schemes. Instead, lifetime CVD risk, treatment benefit, comorbidities, patient preferences, frailty, and potential risk modifiers should be considered. The most important risk modifiers represent psychosocial stressors, an individual’s coronary calcium score and ethnicity. Lately, a series of biomarkers, genetic risk scores, and CV imaging modalities have gained some attention to establish an individual’s CV risk. However, the utility of such tests remains to be assessed in future studies [2].
Individualized lipid management: persons at greatest risk should attain the lowest LDL-C levels
The risk-based, stepwise goals for CVD prevention are summarized in Table 3. While stopping smoking rapidly reduces CVD risk and is the most cost-effective strategy for ASCVD prevention, pharmacological interventions to meet high and very high-risk categories’ targets for BP, LDL-C, and, in patients with DM, blood glucose are usually recommended to reduce an individual’s CVD risk [2].
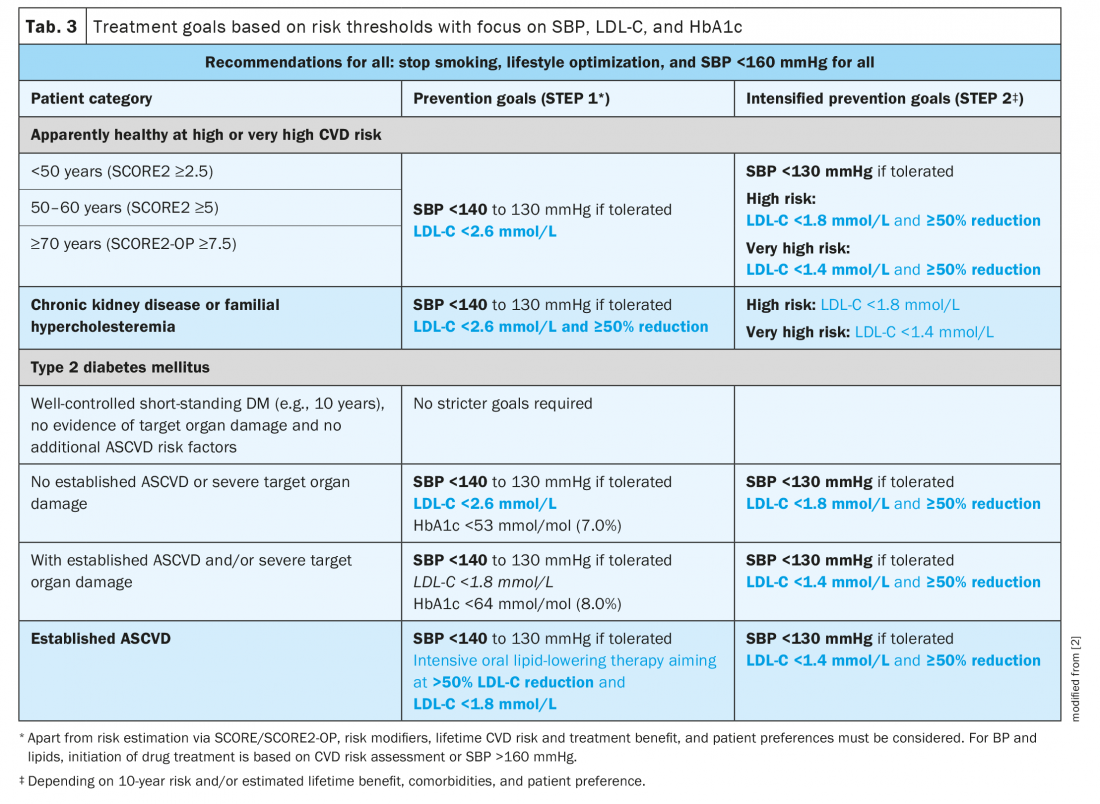
ESC Guideline recommendations for pharmacological lipid lowering: The specific lipid-lowering treatment recommendations for the various patient cohorts are highlighted in Table 4. As there is considerable individual variability in the response to drug treatments, LDL-C monitoring is recommended at baseline and 4 – 6 weeks after initiation or change of treatment. It is essential that after STEP 1, treatment intensification with STEP 2 is considered in all patients. In specific cases at very high risk, it may be indicated to merge both steps and proceed directly to STEP 2.
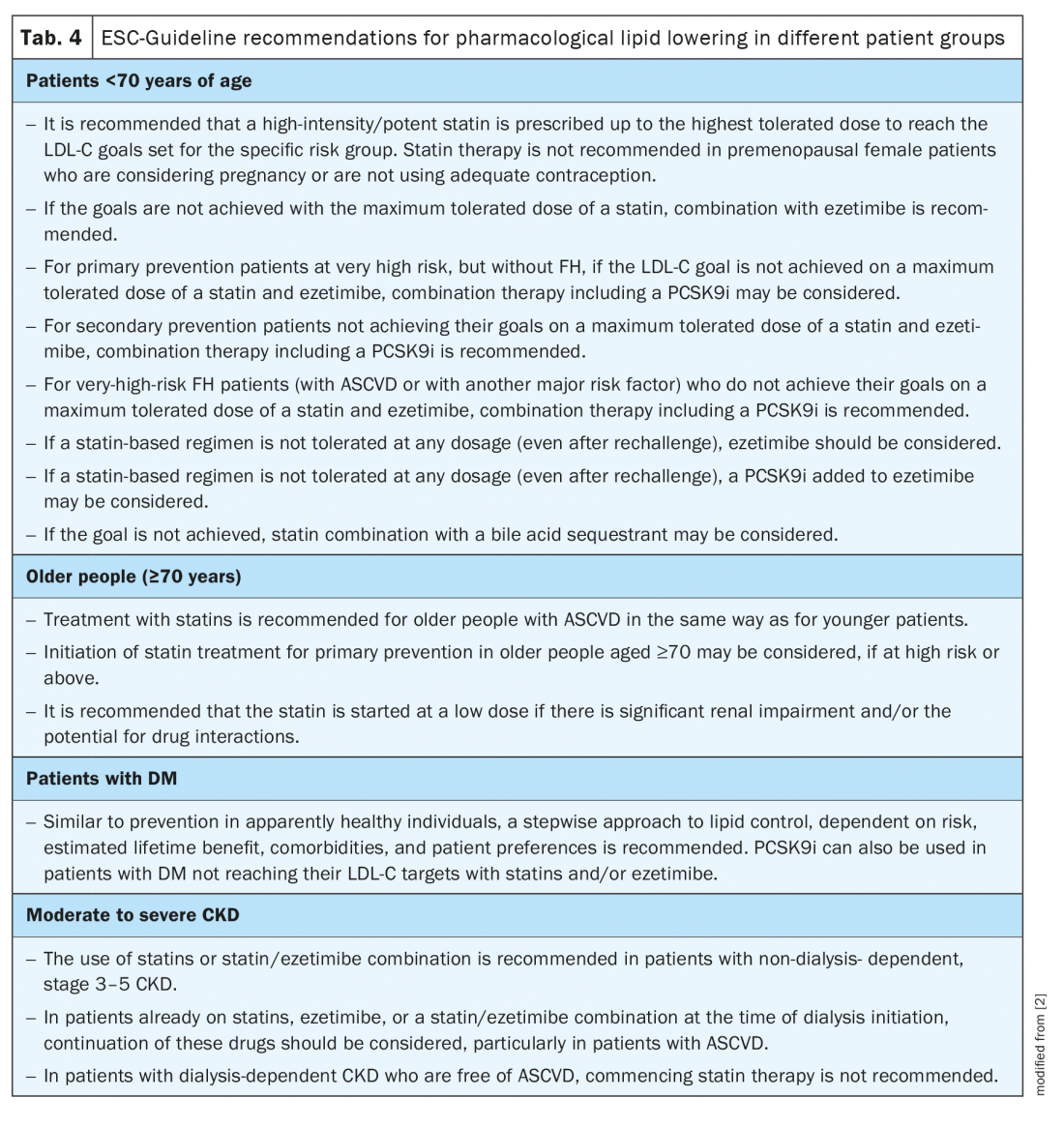
Statins remain the first-choice treatment, achieving LDL-C reductions of ≥50% and reducing the risk of future CV events. Yet, interindividual variations in statin responses warrant monitoring of therapeutic success [4,6]. A cross-sectional observational study across 18 European countries demonstrates that among patients receiving lipid-lowering therapies, fewer than half at high or very high risk achieved their 2016 LDL-C goals, despite optimal use of statins [19]. Approximately one fifth of those patients achieved the even lower 2019 ESC goals [19]. Following the credo “lower is better”, the ESC encourages intensification of treatment by combination of statins with the cholesterol absorption inhibitor ezetimibe and/or a proprotein convertase subtilisin/kexin type 9 inhibitor (PCSK9i) such as evolocumab or alirocumab (Tab. 4) [2]. The expected LDL-C reductions in response to established lipid-lowering drugs are summarized in Table 1.
Take-Home-Messages
- Lowering LDL-C reduces ASCVD risk proportionally to the absolute achieved reduction in LDL-C, with no lower limit at which preventive benefit subsides.
- In the new ESC Guidelines on cardiovascular disease prevention in clinical practice, risk factors are treated in a stepwise risk-based approach to reach the ultimate treatment goals in several categories of individuals, such as apparently healthy people, patients with established ASCVD, and patients with DM.
- 10-year CVD risk is estimated in apparently healthy people aged 40–69 years with SCORE2, and in people aged ≥70 years with SCORE2-OP.
- Lowering LDL-C with statins, ezetimibe, and – if needed – PCSK9 inhibitors, decreases the risk of ASCVD proportionally to the absolute achieved reduction in LDL-C.
- Research indicates that PCSK9-directed antibodies stabilize atheroma and increase fibrous cap thickness.
- Bempedoic acid and the siRNA inclisiran are promising additions in the arsenal of lipid-lowering therapies and await validation in ongoing CV outcome trials.
Literature:
- www.bfs.admin.ch/bfs/de/home/statistiken/gesundheit/gesundheitszustand/krankheiten/herz-kreislauf-erkrankungen.html.
- Visseren FL, et al.: 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With the special contribution of the European Association of Preventive Cardiology (EAPC). European heart journal 2021; 42(34): 3227–3337.
- Zellweger U, et al.: Prevalence of chronic medical conditions in Switzerland: exploring estimates validity by comparing complementary data sources. BMC public health, 2014; 14(1): 1–12.
- Mach F, et al.: 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020; 41(1): 111–188.
- Ference BA, et al.: Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2017; 38(32): 2459–2472.
- Baigent C, et al.: Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010; 376(9753): 1670–1681.
- Cannon CP, et al.: Ezetimibe added to statin therapy after acute coronary syndromes. New England Journal of Medicine 2015; 372(25): 2387–2397.
- Sabatine MS, et al.: Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med 2017; 376(18): 1713–1722.
- Schwartz GG, et al.: Alirocumab and cardiovascular outcomes after acute coronary syndrome. New England Journal of Medicine 2018; 379(22): 2097–2107.
- Prävention der Atherosklerose 2020. Übersicht zu den Empfehlungen der Arbeitsgruppe Lipide und Atherosklerose (AGLA) der Schweizerischen Gesellschaft für Kardiologie (SGK) sowie der European Society of Cardiology (ESC) und der European Atherosclerosis Society (EAS). www.agla.ch/de/empfehlungen, letzter Zugriff: 06.07.2022.
- Cosentino F, et al.: 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. European heart journal 2020; 41(2).
- Cardiology E.S.o., ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018; 39: 3021–3104.
- Wilson PW, et al.: Prediction of coronary heart disease using risk factor categories. Circulation, 1998. 97(18): 1837–1847.
- Woodward M, Brindle P, Tunstall-Pedoe H: Adding social deprivation and family history to cardiovascular risk assessment: the ASSIGN score from the Scottish Heart Health Extended Cohort (SHHEC). Heart 2007; 93(2): 172–176.
- Hobbs F, et al.: 2016 European Guidelines on cardiovascular disease prevention in clinical practice. European heart journal 2016; 37(29).
- Arnett DK, et al.: 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019; 140(11): e596–e646.
- ESC Cardiovasc Risk Collaboration SCORE2 working group. SCORE2 risk prediction algorithms: new models to estimate 10-year risk of cardiovascular disease in Europe. European Heart Journal 2021; 42(25): 2439–2454.
- SCORE2-OP working group and ESC Cardiovascular risk collaboration. SCORE2-OP risk prediction algorithms: estimating incident cardiovascular event risk in older persons in four geographical risk regions. European Heart Journal 2021; 42(25): 2455–2467.
- Ray KK, et al.: EU-Wide Cross-Sectional Observational Study of Lipid-Modifying Therapy Use in Secondary and Primary Care: the DA VINCI study. European journal of preventive cardiology 2021; 28(11): 1279–1289.
- Ray KK, et al.: Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. New England journal of medicine 2020; 382(16): 1507–1519.
- Ray KK, et al.: Safety and efficacy of bempedoic acid to reduce LDL cholesterol. New England Journal of Medicine 2019; 380(11): 1022–1032.
- Zanchin C, et al.: Effects of the PCSK9 antibody alirocumab on coronary atherosclerosis in patients with acute myocardial infarction: a serial, multivessel, intravascular ultrasound, near-infrared spectroscopy and optical coherence tomography imaging study–Rationale and design of the PACMAN-AMI trial. American Heart Journal 2021; 238: 33–44.
- Nicholls SJ, et al.: Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. Jama 2016; 316(22): 2373–2384.
- Nicholls SJ, et al.: Effect of Evolocumab on Coronary Plaque Phenotype and Burden in Statin-Treated Patients Following Myocardial Infarction. JACC: Cardiovascular Imaging, 2022.
- Coppinger C, et al.: A Comprehensive Review of PCSK9 Inhibitors. Journal of Cardiovascular Pharmacology and Therapeutics 2022; 27: 10742484221100107.
- Oyama K, et al.: Effect of evolocumab on acute arterial events across all vascular territories: results from the FOURIER trial. European Heart Journal 2021; 42(47): 4821–4829.
CARDIOVASC 2022; 21(3): 14–19











