Approximately 2-4% of the general population is affected by chronic rhinosinusitis with nasal polyps (CRSwNP) [1]. Treatment options were very limited for a long time, but since the approval of biologics for this indication, new therapeutic horizons have opened up.
The symptoms of CRSwNP can significantly affect quality of life and lead to high levels of distress. The extent of the limitations is about on par with heart failure or asthma [2]. Negative influencing factors include asthma, sensitivity to acetylsalicylic acid and nonsteroidal analgesics, colonization with Staphylococcus aureus, and allergic diseases [2]. Until recently, the range of therapeutic options was small: treatment with intranasal corticosteroids represents the permanent and basic drug therapy. However, in the absence of success, the only remaining option was escalation with systemic steroids [3]. High-dose continuous therapy with systemic steroids increases the risk of osteoporosis, diabetes, cardiovascular events, and infections [3]. In case of lack of response to therapy, the option of surgical intervention remained as an alternative. However, endoscopic sinus surgery is associated with very high recurrence rates, especially for nasal polyps, particularly in those patients who undergo surgery for these polyps [4]. Nearly one in five (18.6%) required at least one additional surgical procedure [4]. A gold standard for assessing disease control in chronic rhinosinusitis does not yet exist, but this would be desirable from both a clinical and research perspective [2]. Meanwhile, biologics have entered the treatment for CRSwNP. In September 2021, mepolizumab (Nucala®) became the first and only anti-interleukin (IL)-5 antibody to receive approval as an add-on therapy to intranasal corticosteroids for severe chronic rhinosinusitis with nasal polyps [5].
IL-5 with a decisive role
In CRSwNP, there is usually mast cell infiltration and Th2 polarization in the airway mucosa in addition to eosinophilia [7]. Eosinophils play a particularly important role in treatment-refractory nasal polyps [1,8–10]. Up to 90% of cases of recurrent nasal polyps are a result of eosinophilic inflammation [8,11,12]. IL-5 is the key cytokine to promote growth, differentiation, survival and recruitment of eosinophils in nasal polyps [9,10,13]. Patients with elevated eosinophil levels were 3Å~ more likely to have a recurrence after nasal polyp surgery than patients with lower eosinophil levels [8]. CRSwNP is a common comorbidity in severe eosinophilic asthma (SEA) and approximately 40% of patients with severe asthma are affected by comorbid nasal polyps [1,14]. Similarly, the occurrence of CRSwNP is associated with the severity of asthma: In mild asthma, only 10-30% have CRSwNP, whereas in severe cases, 70-90% of patients are affected [15]. From an analysis by Howarth et al. indicates that the anti-IL-5 antibody mepolizumab shows improved efficacy as an add-on therapy in patients with SEA and comorbid nasal polyps [16]. In this group of patients, the exacerbation rate was reduced by 80% compared with placebo (vs. 49% in patients without nasal polyps). A recently published study demonstrated that inhibition of IL-5 reduces eosinophilic inflammation as well as the size of nasal polyps [1]. In refractory patients in whom previous therapy, including surgery, failed to improve symptoms, the monoclonal antibody mepolizumab has shown symptomatic improvement with good tolerability as an add-on therapy [1]. These findings are based on the phase III SYNAPSE (StudY in NAsal Polyp patients to assess the Safety and Efficacy of mepolizumab) study, which evaluated the efficacy of mepolizumab vs. placebo as an add-on therapy to intranasal mometasone furoate over a 52-week period [1]. Included patients had to have severe CRSwNP symptomatology (VAS* score >7) as well as have undergone at least one nasal polyp surgery in the past 10 years. The primary endpoints were endoscopic nasal polyp score and VAS score nasal obstruction [1].
Significant improvements
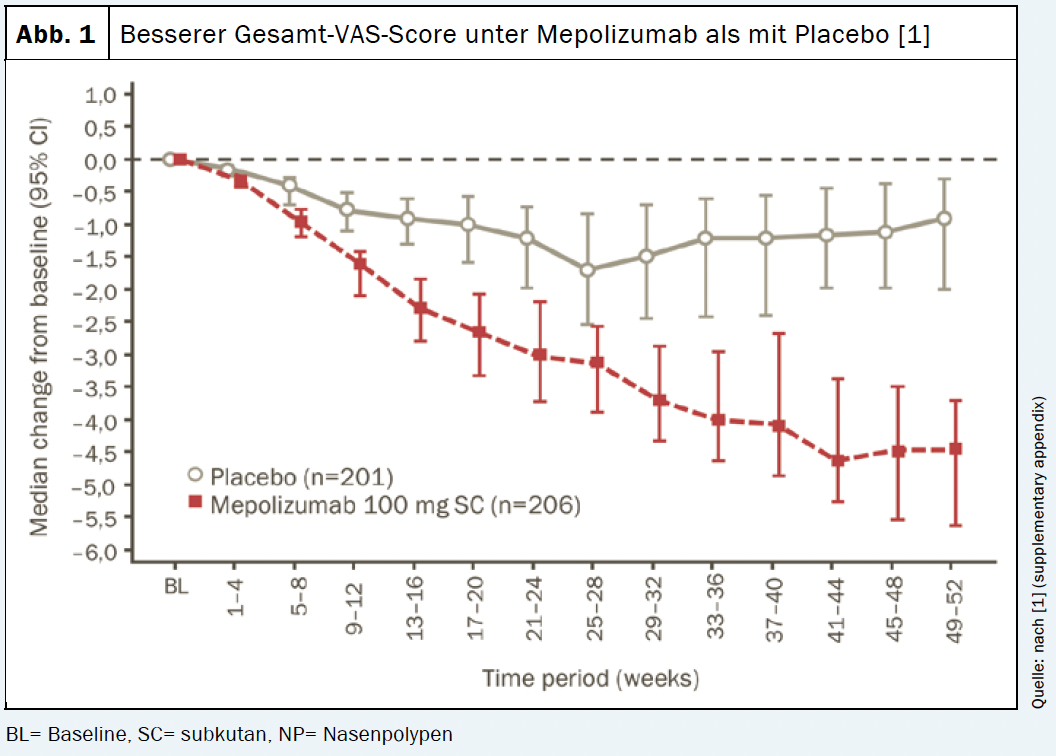
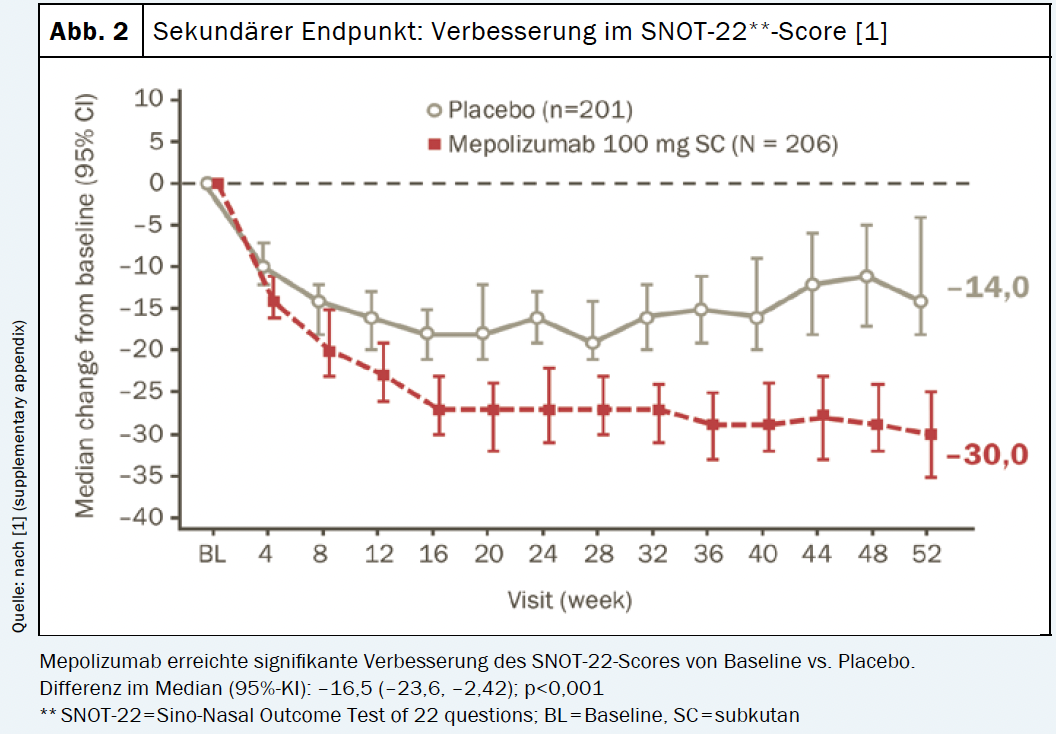
Both the primary and secondary endpoints of the study were met. The mepolizumab arm (n=206) showed significantly greater improvements in endoscopic nasal polyp total score at week 52 and VAS score for nasal obstruction at weeks 49-52 compared with placebo (n=201) [1]. Similarly, time to first nasal polyp surgery proved significantly longer with mepolizumab treatment vs. placebo treatment. The risk of needing surgery within the 12-month study period was reduced by 57% compared with the placebo group [1]. 145 patients (70%) from the mepolizumab arm achieved an improvement of >1 point in the VAS score for total symptoms versus 99 patients (49%) from the placebo group. The response with the IL-5 antibody persisted throughout the study period (Fig. 1). Similarly, a significant improvement was observed in the SNOT†-22 score with a reduction of 30 points compared with baseline (Fig. 2). In addition, the use of systemic steroids was significantly reduced under mepolizumab [1]. In summary, for patients with CRSwNP, mepolizumab offers a targeted add-on treatment option to improve clinical symptoms and reduce the use of systemic corticosteroids. Moreover, in refractory patients, a reduction in the need for repeat nasal polyp surgery can be achieved [1].

Professionals can request the mentioned references from GSK AG.
Imprint
Text/Editorial: Jens Dehn, Mirjam Peter
Source: Hopkins C: Mepolizumab for nasal polyps – Current evidence in the context of clinical practice. Workshop Interlaken, November 12, 2021. Trademarks Disclaimer: Trademarks are owned by or licensed to the GSK group of companies.
©2021 GSK group of companies or its licensor. GlaxoSmithKline AG, Talstrasse 3-5, 3053 Münchenbuchsee, Switzerland
This article was written with the financial support of GlaxoSmithKline Schweiz AG.
© Prime Public Media AG, Zurich 2021
Brief technical information NUCALA
®
:
Nucala (lyophilizate for the preparation of an injection solution for s.c. administration, injection solution in a ready-to-fill pen for s.c. administration) administration, injection solution in a pre-filled pen for s.c. administration, injection solution in a pre-filled syringe for s.c. administration Injection solution in a pre-filled syringe for s.c. administration). Administration). W: Mepolizumab. I: Severe eosinophilic asthma: adjunctive therapeutic agent in adults and adolescents 12 years of age and older with the following criteria: At least. 2 exacerbations in the past 12 months on standard therapy (high-dose ICS and additional maintenance therapy) and/or need for systemic corticosteroids, and blood eosinophil count ≥ 0.15 G/l at treatment initiation or ≥ 0.3 G/l in the past 12 months. Chronic rhinosinusitis with nasal polyps (CRSwNP): adjunctive therapy to intranasal corticosteroids in adults 18 years of age and older with severe CRSwNP that cannot be adequately controlled with intermittent systemic corticosteroids and/or surgical intervention (prefilled pen, prefilled syringe). Eosinophilic granulomatosis with polyangiitis (EGPA): add-on therapy in adults 18 years of age and older with the following criteria: Recurrent or refractory EGPA, previous stabilization of the disease with systemic corticosteroids, or necessary maintenance treatment with systemic corticosteroids and steroid-sparing immunosuppressants, if any. Hypereosinophilia syndrome (HES): adjunctive therapeutic agent for the treatment of adults and adolescents 12 years of age and older with HES without FIP1L1-PDGFRα fusion (F/P negative HES). D: Severe eosinophilic asthma and CRSwNP: 100 mg Nucala s.c. 1Å~ every 4 weeks. EGPA and HES: 300 mg Nucala s.c. 1Å~ every 4 weeks. KI: Hypersensitivity to an ingredient. W/V: Must not be used to treat acute asthma exacerbations. In case of persistent uncontrolled asthma or worsening, physician should be contacted. No abrupt discontinuation of corticosteroids. Local and systemic hypersensitivity reactions (immediate and delayed type) may occur under Nucala. Treat any helminth infestation prior to administration of Nucala. IA: There are no studies on interactions with other drugs. S/S: Pregnancy: Do not use unless clearly necessary. Lactation: Discontinue breastfeeding or therapy with Nucala during lactation. UW: Very common: headache. Common: local reactions (including pain, erythema, itching, burning), eczema, fever, stuffy nose, pharyngitis, lower respiratory tract infections, urinary tract infections, upper abdominal pain, back pain. HES study: very common: infections. Post-marketing experience: rare: Hypersensitivity (including anaphylaxis). P: Lyophilisate for the preparation of a solution for injection for s.c. use. Administration: 100 mg Å~1 vial. Injection solution for s.c. administration. Administration: ready-to-use pen of 100 mg Å~1, ready-to-use syringe of 100 mg Å~1. AK: B. Kassenzul.ssig (Lim). Date of information: November 2020 (lyophilisate), October 2021 (prefilled pen, prefilled syringe). GlaxoSmithKline AG, 3053 Münchenbuchsee. For detailed information, please visit www.swissmedicinfo.ch. Please report adverse drug reactions at pv.swiss@gsk.com. Abbreviations: CRSwnP, severe chronic rhinosinusitis with nasal polyps; EGPA, eosinophilic granulomatosis with polyangiitis; HES, hypereosinophilia syndrome; SEA: severe eosinophilic asthma; VAS, Visual Analogue Scale References: 1. Nucala 10/2021 SmPC. www.swissmedicinfo.ch. 2. waist. C et al. Mepolizumab in a population with severe eosinophilic asthma and corticosteroid dependence results from a French early access program. Eur Respir J 2020; 55 :11. 3, Han JK, Bachert C, Fokkens W et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021; published online April 16. 4 Roufosse F, Kahn JE, Rothenberg ME, et al. Efficacy and safety of mepolizumab in hypereosinophilic syndrome: a phase III, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020. 5 Wechsler ME, et al. Mepolizumab or Placebo for Eosinophilic Granulomatosis with Polyangiitis. N Engl J Med 2017; 376:1921-1932. Professionals can request the above references from GlaxoSmithKline AG. Trade marks are owned by or licensed to the GSK group of companies. ©2021 GSK group of companies or its licensor. GlaxoSmithKline AG, Talstrasse 3-5, 3053 Münchenbuchsee, Switzerland PM-CH-MPL-ADVT-210005-11/2021
Literature:
Han JK, Bachert C, Fokkens W, et al: Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med 2021; 10: 1141-1153.
2 Fokkens WJ, Lund VJ, Hopkins C et al: European Position Paper on Rhinosinusitis and Nasal Polyps 2020. rhinology 2020; Suppl 29: 1-464.
3, Stuck BA, Beule A, Jobst D et al. Guideline for “rhinosinusitis”-long version: S2k guideline of the German College of General Practitioners and Family Physicians and the German Society for Oto-Rhino-Laryngology, Head and Neck Surgery. ENT 2018; 66 (1): 38-74; doi: 10.1007/s00106-017-0401-5.
4 Loftus CA, Soler ZM, Koochakzadeh S, et al: Revision surgery rates in chronic rhinosinusitis with nasal polyps: meta-analysis of risk factors. Int Forum Allergy Rhinol 2020; 10 (2): 199-207.
5. Professional Information Nucala, 10/2021, www.swissmedicinfo.ch
6. equals GJ: Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol 2000; 105(4): 651-663.
7th S2k Guideline Rhinosinusitis, AWMF Register No. 017 /049 and 053-012, www.awmf.org/uploads/tx_szleitlinien/017-049_und_053-012l_S2k_Rhinosinusitis_2019-04.pdf
Tosun F, et al: Relationship between postoperative recurrence rate and eosinophil density of nasal polyps. Ann Otol Rhinol Laryngol. 2010; 119(7): 455-459.
9 Bachert C, Gevaert P, Holtappels G, et al: Total and specific IgE in nasal polyps is related to local eosinophilic inflammation. J Allergy Clin Immunol 2001; 107(4): 607-614.
10. Bachert C, Wagenmann M, Hauser U, Rudack C: IL-5 synthesis is upregulated in human nasal polyp tissue. J Allergy Clin Immunol 1997; 99(6.1): 837-842.
11. Lou H, Meng Y, Piao Y, et al: Cellular phenotyping of chronic rhinosinusitis with nasal polyps. Rhinology 2016; 54: 150-159.
12 Fujieda S, Imoto Y, Kato Y, et al: Eosinophilic chronic rhinosinusitis. Allergol Int. 2019;68(4): 403-412.
13 Hulse KE, et al: Pathogenesis of nasal polyposis. Clin Exp Allergy 2015; 45(2): 328-346.
14 Harrison T, et al: Real-world mepolizumab in the prospective severe asthma REALITI-A study: initial analysis. Eur Respir J 2020; 56(4): 200015.
15. Klimek L, Förster-Ruhrmann U, Becker S, et al: Position paper: Application of biologics in chronic rhinosinusitis with polyposis nasi (CRSwNP) in the German health care system – Recommendations of the Association of German Allergists (AeDA) and the WGs Clinical Immunology, Allergology and Environmental Medicine and Rhinology and Rhinosurgery of the German Society of Otolaryngology, Head and Neck Surgery (DGHNOKHC). Laryngorhinootology 2020; 99(08): 511-527.
16 Howarth P, Chupp G, Nelsen LM et al: Severe eosinophilic asthma with nasal polyposis: A phenotype for improved sinonasal and asthma outcomes with mepolizumab therapy. J Allergy Clin Immunol 2020; 145: 1713-1715.












