The Th2 response of atopic dermatitis is strongly controlled by IL-13, which activates the type II receptor by binding to the IL-13Rα chain. IL-13 is expressed at higher levels than IL-4 in the lesional skin of patients with atopic dermatitis, and its direct skin damaging effects are increasingly well known. By targeting these pathomechanisms, we are moving toward the goal of individualized precision medicine.
Dermatology, like the skin itself, is constantly changing. Like everywhere else in medicine, our understanding of basic pathophysiological processes has expanded dramatically and we are learning every day. For a long time, the only treatment modality for atopic dermatitis consisted predominantly of the use of highly potent steroids, supplemented at most by topical calcineurin inhibitors, phototherapy and, in severe forms, immunosuppressants such as cyclosporin A. This approach can now be increasingly extended through a much more targeted use of pharmaceuticals thanks to our more detailed understanding of the underlying pathology [1].
Atopic dermatitis, like alopecia areata or bronchial asthma, is one of the type 2 dominant diseases caused by complex interactions between genetics and environment. Nevertheless, commonalities between clinically very different phenotypes can be identified. Some interleukins and their receptors, as well as downstream signaling pathways, have been shown to be of central importance.
The origin of the T2 immune response is thought to be protection against parasitic infections. In addition to the classical type 2 T helper cells (Th2 cells), the sources of the essential cytokines IL-4, IL-13, and IL-5 include innate type 2 lymphoid cells (ILC-2), mast cells, and basophils. Also, IL-5 plays a key role in the maturation and recruitment of eosinophils in most tissues, where they act as effector cells of allergic reactions [2].
Molecular mode of action
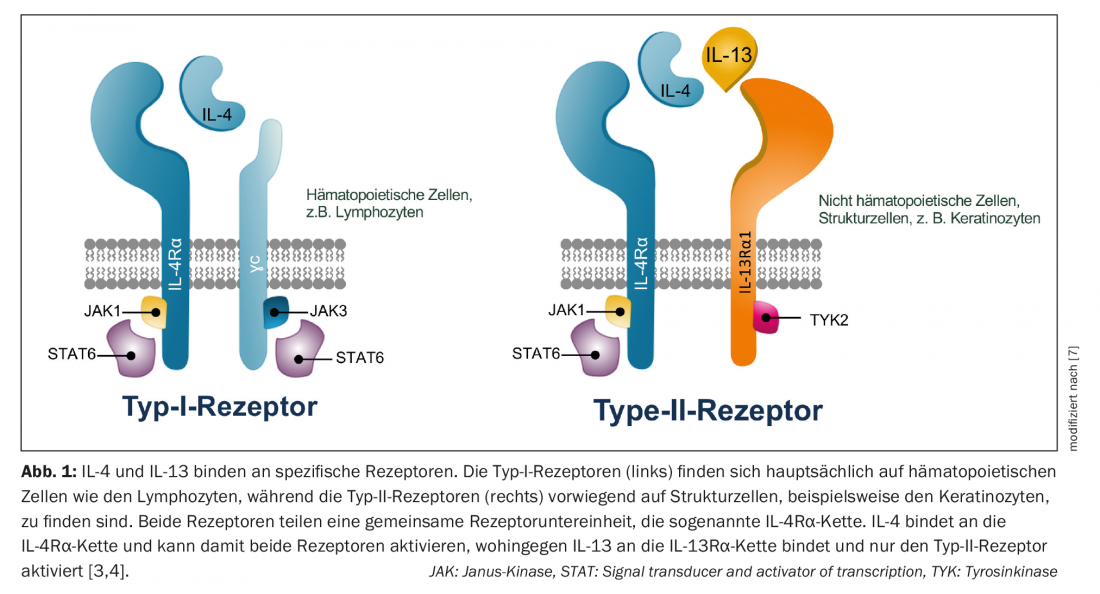
IL-4 and IL-13 bind to specific receptors. Type I receptors are mainly expressed in lymph nodes and play a role in the humoral immune response, whereas type II receptors mediate the immune response in peripheral tissues and therefore in the skin. IL-4 can activate both receptors, whereas IL-13 activates only the type II receptor [3]. Both IL-13 and IL-4 bind to the so-called type II receptor, consisting of the IL-4Rα subunit and the IL-13Rα1 subunit. In this process, the two cytokines compete for binding to the type II receptor – therefore, the ratio of IL-4/IL-13 present determines which of the two cytokines controls the inflammatory response [4] (Fig. 1). In addition, IL-13 binds to the IL-13Rα2 receptor, but its role is not yet fully understood [5]. The Th2 response of atopic dermatitis is probably not predominantly controlled by IL-4 as previously thought, but rather mainly by IL-13 via the type II receptor [6–8].
Expression and disease progression of IL-13
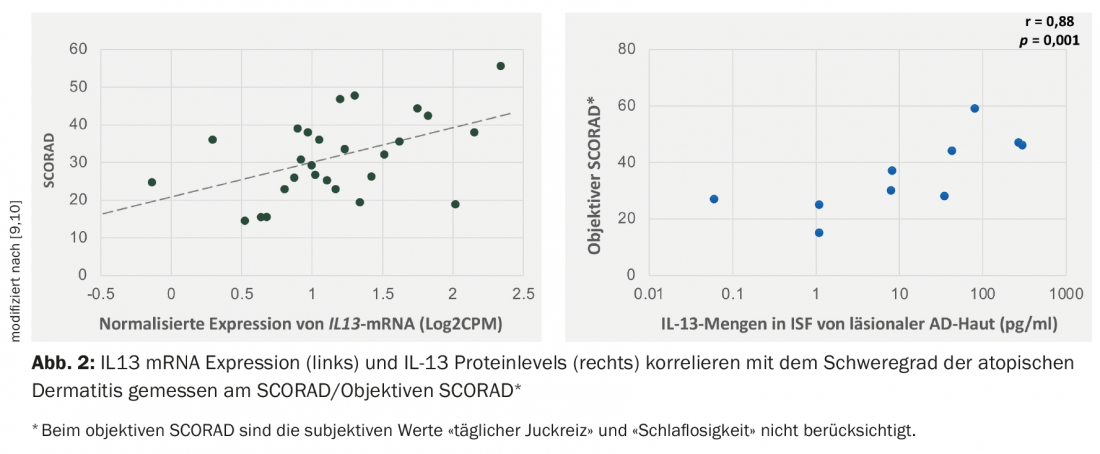
Studies have shown that IL-13 is expressed to a higher degree than IL-4 in the lesional skin of patients with atopic dermatitis, and the level of IL13 mRNA expression correlates positively with the index of severity (SCORAD, SCORing Atopic Dermatitis) [9–11] (Fig. 2).
Intracellular signaling cascade
Targeting the signaling pathways involved in disease is the goal of precision medicine (see section below). Binding of IL-13 and IL-4 to their receptors activates downstream kinases that phosphorylate each other. To the type II receptor, IL-4 binds via the IL-4Rα chain and IL-13 binds via the IL-13Rα chain and activates Janus kinase (JAK) 1 and tyrosine kinase 2 (TYK2). Binding of IL-4 to the type I receptor activates JAK1 and JAK3 [4]. IL-5, which is important for the recruitment of eosinophils as key effector cells in allergy, also activates JAK2 [12]. Phosphorylation of the respective JAKs activates the transcription factor STAT6 (signal transducer and activator of transcription 6), initiating gene expression in the nucleus. Since JAKs are involved in a variety of signaling cascades via different receptor types, their blockade by JAK inhibitors (JAKis) is another possible and effective, but less specific, therapeutic approach [13].
Effects of IL-13 overexpression on the skin.
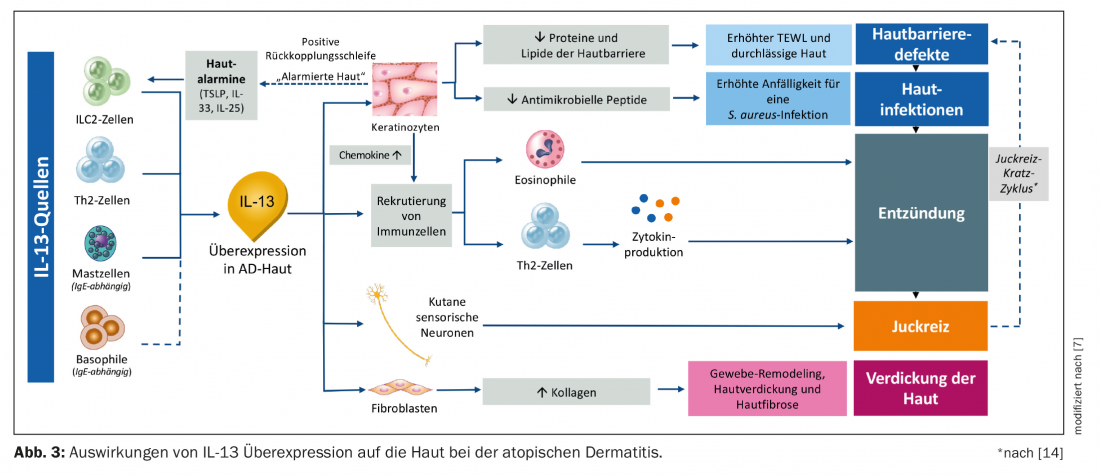
In atopic dermatitis, IL-13 is increased by mast cells, Th2, ILC-2, and basophil cells. Keratinocytes secrete so-called alarmins (TSLP, IL-33, IL-25) and thus stimulate ILC-2 cells to secrete IL-13 and IL-5. At the same time, this decreases the secretion of antimicrobial peptides and lipids, which makes the skin barrier more permeable and results in increased susceptibility to S. aureus and skin inflammation. IL-13 overproduction also alerts the immune system, resulting in increased production of eosinophils and Th2 cells and cytokine-mediated inflammation of the tissue (Fig. 3) [7]. In addition, other cytokines such as IL-10, IL-22, and IL-31 have been implicated [8].
The increased itching is due to the repeated stimulation of cutaneous sensory neurons, which secrete increased IL-4 and IL-13 [7]. Another cytokine with highly pruritogenic potential is IL-31 [15,16]. The release of these substances leads to an itch-scratch cycle, which further increases the permeability of the skin barrier. The feedback loop that is then triggered causes the keratinocytes to go on alert again and produce more skin alarmins, which again stimulate the ILC-2 cells to produce even more IL-13. Through this cycle, the signaling cascade is re-initiated and the disease state worsens. In addition, fibroblasts are stimulated to reproduce via IL-13; collagen production is increased, which may result in skin thickening and additional skin fibrosis. In the longer term, this leads to chronic lichenification of the skin [5,17–22].
Therapeutic strategies
Atopic dermatitis is a disease strongly controlled by IL-13. The following therapeutic strategies to inhibit the biological activity are possible [7]:
- small molecules that influence the interaction between IL-13 and receptor: Protein-Protein Interaction Modulators (PPIMs).
- small molecules that affect downstream intracellular signaling pathways (kinase inhibitors, JAKis).
- Biologics that inhibit signaling through IL-13, for example by blocking its binding to the receptor.
PPIMs are currently the subject of research; they are designed using molecular libraries and dynamic simulations. This in silico approachallows the development of new drugs targeting specific interleukins and their receptors [7,23].
JAKis with different selectivity are already in use in the treatment of rheumatoid arthritis. Oral and topical applications are possible for the treatment of atopic dermatitis, and the onset of action is very rapid. Due to their mode of action, JAKis partially inhibit several cytokines, e.g. IL4, IL-5, IL-31 and other cytokines in addition to IL-13 – in contrast to biologics, which only inhibit individual cytokines almost completely. The first-generation JAKis inhibit mainly JAK1 and JAK2 (ruxolitinib and baricitinib) or all JAKs to a similar extent (pan-JAKis delgocitinib, tofacitinib, peficitinib, oclacitinib) [13,24–27]. Baricitinib is already approved in Switzerland for the treatment of adult patients with moderate-to-severe AD [28]. A veterinary marketing authorization exists for oclacitinib for the treatment of AD in dogs [29]. The second generation of JAKis are somewhat more selective and act primarily, but not exclusively, on JAK1 (abrocitinib, filgotinib, upadacitinib, itacitinib) or TYK2 (BMS-986165) [25–27]. The safety and tolerability profiles of the various JAKis differ depending on the dosage form and molecule. Overall, upper respiratory tract infections have been observed as the most common side effect; in addition, an increase in creatine phosphokinase and liver transaminases may be measured. In addition, an increased risk for the occurrence of herpes zoster is a possible side effect [25,28].
A first-generation biologic for the treatment of atopic dermatitis is dupilumab, a subcutaneously administered monoclonal antibody that binds to the IL-4Rα subunit of type I and type II receptors. Thus, the signaling mediated by IL-4 and IL-13 is prevented by receptor types I and II. Dupilumab has been approved for the treatment of patients with moderate to severe atopic dermatitis in Switzerland since 2019 [30]. It has very good efficacy and a favorable tolerability profile. In addition to atopic dermatitis, it can also be used for various other conditions such as severe forms of bronchial asthma and chronic rhinosinusitis with nasal polyps. Note the occurrence of transient eosinophilia and increased incidence of conjuntivitis as the most common associated symptoms; other side effects or changes in laboratory parameters are very rare [2,31–33].
Precision medicine through selective inhibition of IL-13
Targeted and specific blocking of molecular signaling pathways is the goal of precision medicine. The development of PPIMs will probably lead the way here. To specifically inhibit IL-13, the biologics lebrikizumab and tralokinumab have been developed to date, both of which have been previously studied in atopic dermatitis [34]. Lebrikizumab selectively inhibits the formation of heterodimeric receptor signaling complexes of IL-13Rα1 and IL-4Rα [35]. Phase IIb studies showed rapid, dose-dependent effects on various clinical parameters in atopic dermatitis [36]. Tralokinumab is a fully human IgG4 monoclonal antibody that binds with very high affinity to IL-13. The epitope overlaps with the binding site of IL-13Rα receptors, preventing binding of IL-13 to both IL-13Rα1 and IL-13Rα2 [37]. Thus, an important driver of the pathogenesis of atopic dermatitis is selectively eliminated.
It has been postulated that this strategy may be more effective than blocking IL-13Rα1 binding alone [9]. In clinical trials in patients with moderate to severe atopic dermatitis, tralokinumab in combination with steroids resulted in rapid and sustained improvement in AD symptoms for at least 52 weeks. The EASI, SCORAD index, dermatological quality of life index (DLQI), and pruritus numerical rating scale had significantly better scores compared with the placebo group [2,38–40]. In addition, a good tolerability and safety profile, was demonstrated, also with transient measurable eosinophilia and slightly increased incidence of conjunctivitis, with a concomitant reduction in skin infections compared to the control group [7,39,40].
Outlook
Atopic dermatitis is characterized by a wide spectrum of clinical phenotypes. We are increasingly coming to the conclusion that complex basal, genetic and immunopathogenetic mechanisms underlie this disease. The absence of a single, all-explanatory factor complicates the therapeutic response and requires individualized therapy. Tailored treatment is the challenge and goal of patient-centered medicine. IL-13 is expressed at higher levels than IL-4 in the lesional skin of patients with atopic dermatitis, and its direct skin-damaging effects are increasingly well known. The better we succeed in specifically influencing these pathomechanisms, the closer we will come to the goal of individualized precision medicine, also in atopic dermatitis.
Take-Home Messages
- Sources of cytokines IL-4, IL-13, IL-5 are Th2 cells and ILC-2 cells, mast cells and basophils.
- IL-4 binds to type I and type II receptors.
- IL-13 binds only to type II receptors found in the skin.
- The Th2 response of atopic dermatitis is strongly controlled by IL-13, which activates the type II receptor by binding to the IL-13Rα chain.
- IL-13 is expressed at higher levels than IL4 in patients with atopic dermatitis and correlates with disease severity.

Literature:
- Siegels D, Heratizadeh A, Abraham S, European Academy of Allergy, Clinical Immunology Atopic Dermatitis Guideline group, et al: European Academy of Allergy, Clinical Immunology Atopic Dermatitis Guideline group. Systemic treatments in the management of atopic dermatitis: A systematic review and meta-analysis. Allergy. 2021;76: 1053-1076.
- Gandhi NA, Bennett BL, Graham NM, et al: Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov. 2016;15: 35-50.
- Mueller TD, Zhang JL, Sebald W, Duschl A: Structure, binding, and antagonists in the IL-4/IL-13 receptor system. Biochim Biophys Acta. 2002;1592(3): 237-250.
- McCormick SM, Heller NM: Commentary: IL-4 and IL-13 receptors and signaling. Cytokines. 2015; 75: 38-50.
- Bao K, Reinhardt RL: The differential expression of IL-4 and IL-13 and its impact on type-2 immunity. Cytokines. 2015; 75: 25-37.
- Leung DY, Bieber T: Atopic dermatitis. Lancet 2003; 361: 151-160. [PubMed: 12531593]
- Bieber T: Interleukin-13: Targeting an underestimated cytokine in atopic dermatitis. Review. Allergy 2020; 75: 54-62.
- Langan SM, Irvine AD, Weidinger S: Atopic dermatitis. Lancet. 2020; 396: 345-360.
- Tsoi J, Rodriguez E, Sarkar MK, et al: Atopic dermatitis is an IL-13 dominant disease with greater molecular heterogeneity compared to psoriasis. J Invest Dermatol 2019;139: 1480-1489.
- Szegedi K, Lutter R, et al: Cytokine profiles in interstitial fluid from chronic atopic dermatitis skin. JEADV 2015; 29: 2136-2144.
- Ungar B, Garcet S, Gonzalez J, et al: An integrated model of atopic dermatitis biomarkers highlights the systemic nature of the disease. J Invest Dermatol 2017; 137: 603-613.
- Long H, Liao W, Wang L, Lu Q: A player and coordinator: the versatile roles of eosinophils in the immune system. Transfus Med Hemother. 2016; 43: 96-108.
- Mobasher P, Heydari Seradj M, Raffi J, et al: Oral small molecules for the treatment of atopic dermatitis: a systematic review. J Dermatolog Treat. 2018; 201: 1-8.
- Mack MR, Kim BS: The Itch-Scratch Cycle: A Neuroimmune Perspective. Trends Immunol 2018; 39: 980-991.
- Datsi A, Steinhoff M, Ahmad F, et al: Interleukin-31: The “itchy” cytokine in inflammation and therapy. Allergy. 2021 Feb 24. doi: 10.1111/all.14791. epub ahead of print.
- Dillon SR, Sprecher C, Hammond A, et al; Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol 2004;5: 752-760.
- Salimi M, Barlow JL, Saunders SP, et al: A role for IL-25 and IL-33- driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med 2013; 210: 2939-2950.
- Moriya C, Jinnin M, Yamane K, et al: Expression of matrix metalloproteinase-13 is controlled by IL-13 via PI3K/Akt3 and PKC-d in normal human dermal fibroblasts. J Invest Dermatol 2011; 131: 655-661.
- Howell MD, Kim BE, Gao P, et al: Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol 2007; 120: 150-155.
- Kim BE, Leung DY, Boguniewicz M, Howell MD: Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol 2008; 126: 332-337.
- Berdyshev E, Goleva E, Bronova I, et al. Lipid abnormalities in atopic skin are driven by type 2 cytokines. JCI Insight. 2018;3(4). https://doi.org/10.1172/jci.insight.98006
- Oetjen LK, Mack MR, Feng J, et al: Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell 2017; 171: 217-228.
- Majumdar S, Ghosh A, Saha S: Modulating interleukins and their receptors interactions with small chemicals using in silico approach for asthma. Curr Top Med Chem 2018; 18: 1123-1113.
- Gadina M, Le MT, Schwartz DM, et al: Janus kinases to jakinibs: from basic insights to clinical practice. Rheumatology. 2019;58(suppl 1): i4-i16.
- Rodrigues MA, Torres T: JAK/STAT inhibitors for the treatment of atopic dermatitis. J Dermatolog Treat. 2020; 31: 33-40.
- Cotter DG, Schairer D, Eichenfield L: Emerging therapies for atopic dermatitis: JAK inhibitors. J Am Acad Dermatol. 2018;78(suppl 1): S53-S62.
- Schwartz DM, Kanno Y, Villarino A, et al: JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov 2017: 843-862.
- Specialty Information Olumiant® (baricitinib). www.swissmedicinfo.ch
- Specialized information Apoquel® (oclacitinib). www.tierarzneimittel.ch
- Specialist information Dupixent® (dupilumab). www.swissmedicinfo.ch
- Chang HY, Nadeau KC. IL-4Ralpha inhibitor for atopic disease. Cell. 2017;170:222.
- Beck LA, Thaçi D, Hamilton JD, et al: Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014 Jul 10; 371: 130-139.
- Simpson EL, Bieber T, Guttman-Yassky E, et al: Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016; 375: 2335-2348.
- Gonçalves F, Freitas E, Torres T: Selective IL-13 inhibitors for the treatment of atopic dermatitis. Drugs Context. 2021;10: 1-7.
- Ultsch M, Bevers J, Nakamura G, et al: Structural basis of signaling blockade by anti-IL-13 antibody Lebrikizumab. J Mol Biol. 2013; 425(8): 1330-1339.
- Guttman-Yassky E, Blauvelt A, Eichenfield LF, et al: Efficacy and Safety of Lebrikizumab, a High-Affinity Interleukin 13 Inhibitor, in Adults With Moderate to Severe Atopic Dermatitis: A Phase 2b Randomized Clinical Trial. JAMA Dermatol 2020 Apr 1; 156(4): 411-420.
- Popovic B, Breed J, Rees DG, et al: Structural characterization reveals mechanism of IL-13-neutralizing monoclonal antibody tralokinumab as inhibition of binding to IL-13Ralpha1 and IL-13Ralpha2. J Mol Biol 2017;429: 208-219.
- Wollenberg A, Howell MD, Guttman-Yassky E, et al: Treatment of atopic dermatitis with tralokinumab, an anti-IL-13 mAb. J Allergy Clin Immunol 2019; 143: 135-141.
- Wollenberg A, Blauvelt A, Guttman-Yassky E, et al: Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol 2021; 184: 437-449.
- Silverberg JI, Toth D, Bieber T, et al: Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial. Br J Dermatol 2021; 184: 450-463.
DERMATOLOGIE PRAXIS 2021; 31(4): 10-14