In inflammatory processes of dermatological diseases, pro-inflammatory cytokines play a central role. Therefore they are in focus therapeutic approaches when it comes to breaking critical signaling pathways. In addition to monoclonal antibodies, small-molecule agents directed against Janus kinases (JAK) are increasingly being used. The first approvals of JAK inhibitors have already been received in atopic dermatitis and psoriasis, but data to date are also promising in other inflammatory skin diseases such as vitiligo and alopecia areata.
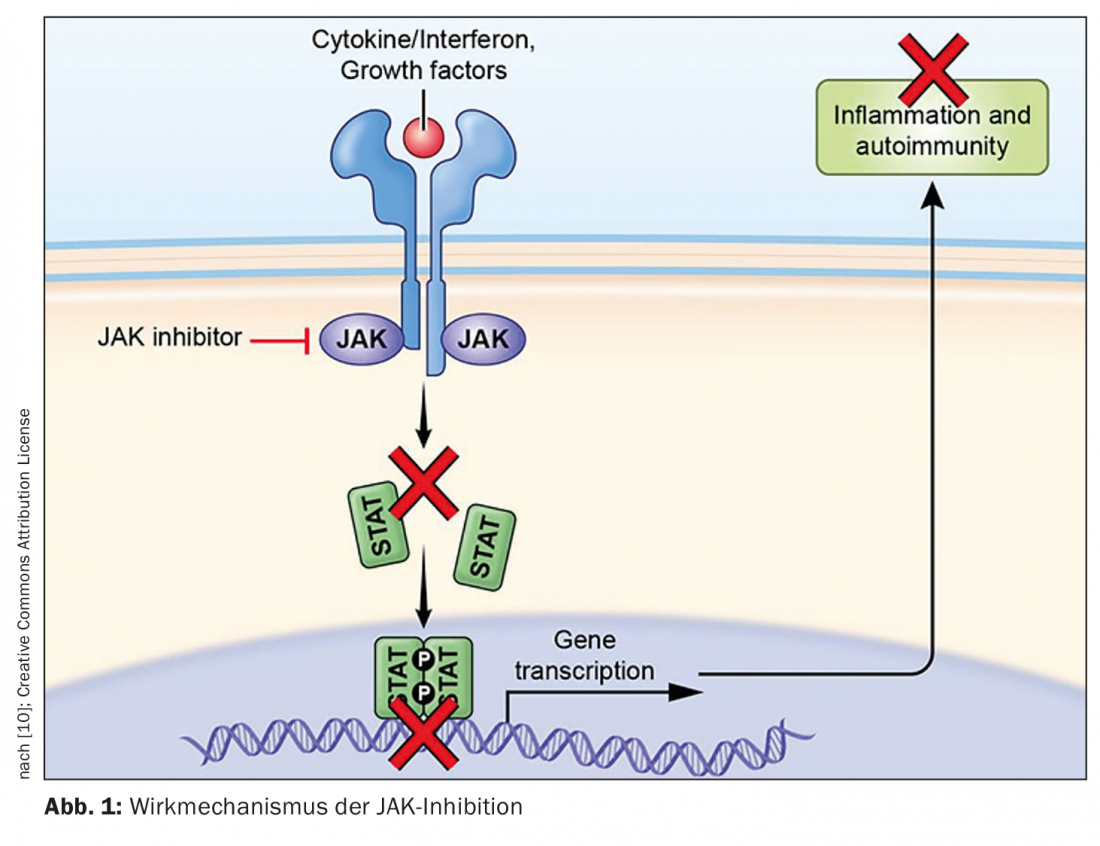
JAK inhibitors represent a valuable addition to systemic treatment strategies for inflammatory skin diseases. “The JAK-STAT signaling pathway is an essential key in the treatment of immunological and chronic inflammatory diseases. If we succeed in stopping pro-inflammatory cytokine cascades, this has an immediate effect on the inflammatory response,” explains Prof. Michael Hertl, MD, acting President of the German Dermatological Society (DDG) and Director of the Clinic for Dermatology and Allergology, Philipps University and University Hospital Marburg [1]. By occupying the STAT docking sites, JAK inhibitors inhibit the JAK-STAT signaling pathway and thus the pro-inflammatory signaling cascade (Fig. 1).
Specific intracellular signaling pathways are modulated
The deregulated and activated Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway plays a key role in the pathogenesis of many inflammatory dermatoses. Four subunits of Janus kinases (JAKs) are present in human cells: JAK1, JAK2, JAK3 and TYK2. Intracellular signal transduction of numerous proinflammatory cytokines is mediated by Janus kinases. After dimerization and autophosphorylation, STAT proteins are recruited and phosphorylated, which control transcription of target genes in the nucleus. Janus kinases form homodimers or heterodimers, with JAK2 also occurring as a homodimer [2]. Pharmacological inhibition of JAKs can downregulate the activity of the JAK-STAT signaling pathway, thereby preventing intracellular signal transduction of several proinflammatory cytokines [1,2].
JAK inhibitors conquer diverse dermatological indication areas
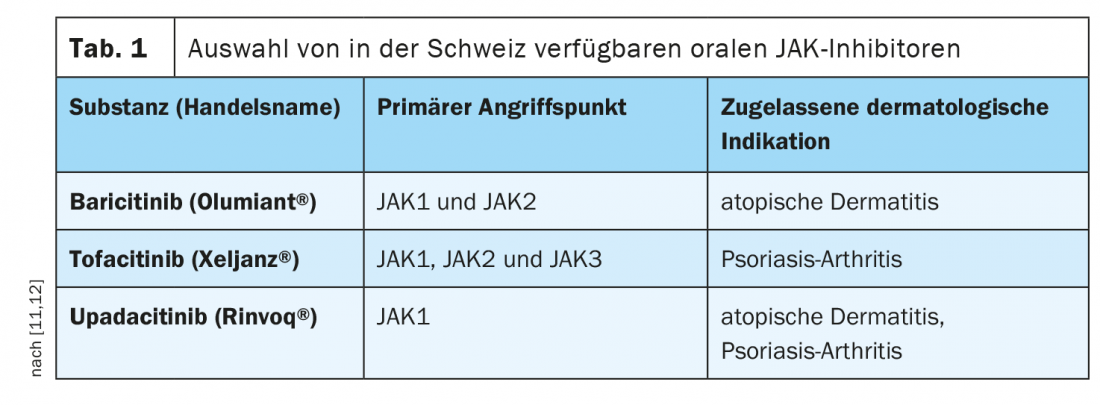
For the treatment of adult patients with moderate to severe atopic dermatitis, baricitinib (Olumiant®) was approved in Switzerland in the first quarter of last year as the first representative from the group of oral JAK inhibitors, and in November upadacitinib (Rinvoq®) also cleared the approval hurdle [4]. Baricitinib in particular shows high potency and selectivity for the JAK1 and JAK2 subtypes, whereas upadacitinib targets JAK1 primarily (Table 1) . Abrocitinib, a third member of the Janus kinase inhibitors, has already been approved in the EU for this indication [5]. For the treatment of adults with active psoriatic arthritis, tofacitinib (Xeljanz®) is approved in Switzerland in combination with a conventional synthetic DMARD. In addition, a phase II study demonstrated the efficacy of TYK2 inhibition using deucravacitinib-BMS986165 compared with placebo [6].
The data for JAK inhibitors also look promising with regard to their potential use in alopecia areata. In a meta-analysis of 30 small trials of JAK inhibitors for alopecia areata, 72.4% of patients responded to therapy, including 45.7% with good (50-100% hair regrowth) and 21.4% with partial response (5-50% hair regrowth) [7]. The double-blind, randomized-controlled ALLEGRO trial also evaluated the efficacy of JAK inhibitors in women and men with alopecia areata. All participants had more than 50 percent hair loss on the scalp at baseline. Treatment for 24 weeks was effective and well tolerated: compared with the placebo group, participants in the verum group had hair loss of 20% or less [8]. The side effect profile of the JAK inhibitors is clear, explains Prof. Hertl. Infections can occur more frequently, for example in the nose and throat or in the respiratory tract. Urinary tract infections, gastrointestinal disorders and acne have also been observed. “When an infection is acute, therapy with JAK inhibitors should be paused, of course,” the DDG president said. However, the expert adds that this works very well due to the oral application and short half-life of the drugs [1]. The latest developments include topically applicable JAK inhibitors. A JAK 1/2 inhibitor administered by cream improved diseased facial skin (repigmentation of vitiligo lesions) in approximately 50% of patients versus 3% with placebo in the treatment of autoimmune-induced vitiligo [9]. “These are promising results suggesting that this cream could be an effective therapeutic option for patients with vitiligo,” emphasized Prof. Hertl, adding, “The side effects known with cortisone ointments were not observed” [1].
Congress: Dermatology compact and practical
Literature:
- “Small molecules against atopic dermatitis, psoriasis, vitiligo and circular hair loss: JAK inhibitors in inflammatory skin diseases expand treatment spectrum,” Deutsche Dermatologische Gesellschaft e. V. (DDG), Jan. 31, 2022.
- Schwartz DM, et al: JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov 2017; 16: 843-862.
- Damsky W, et al: The emerging role of Janus kinase inhibitors in the treatment of autoimmune and inflammatory diseases. J Allergy Clin Immunol 2021; 147: 814-826.
- Drug Information, www.swissmedicinfo.ch, (last accessed Mar. 07, 2022).
- Klein B, Treudler R, Simon JC: JAK inhibitors in dermatology – small molecules, big impact? Overview of mechanism of action, study results, and potential adverse effects. J Dtsch Dermatol Ges 2022; 20(1): 19-25.
- Papp K, et al: Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med 2018; 379: 1313-1321.
- Phan K, Sebaratnam DF: JAK inhibitors for alopecia areata: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol 2019; 33: 850-856.
- King B, et al: A phase 2a randomized, placebo-controlled study to evaluate the efficacy and safety of the oral Janus kinase inhibitors ritlecitinib and brepocitinib in alopecia areata: 24-week results. J Am Acad Dermatol 2021; 85(2): 379-387.
- Rosmarin D, et al: Ruxolitinib cream for treatment of vitiligo: a randomised, controlled, phase 2 trial. Lancet 2020; 396: 110-120.
- Alexander M, et al: Jakinibs of All Trades: Inhibiting Cytokine Signaling in Immune-Mediated Pathologies. Pharmaceuticals 2022; 15(1): 48.
- Bonnekoh H, Butze M, Metz M: Characterization of the effects of new therapies for the treatment of atopic dermatitis on pruritus. J Dtsch Dermatol Ges 2022; 20(2): 150-156,
- Karonitsch T: Place of JAK inhibitors (“small is beautiful”). J Miner Metabolism Musculoskeletal Diseases 2021; 28: 78-83.
DERMATOLOGIE PRAXIS 2022; 32(2): 30-31 (published 4/20/22, ahead of print).