An excessive Th2 immune response is characteristic of atopic dermatitis. Biologics specifically interfere with signaling cascades of Th2 inflammatory processes. Recently developed novel drugs include tralokinumab. Meanwhile, impressive efficacy evidence from various studies is available for this monoclonal antibody directed against interleukin (IL)-13, including promising long-term data.
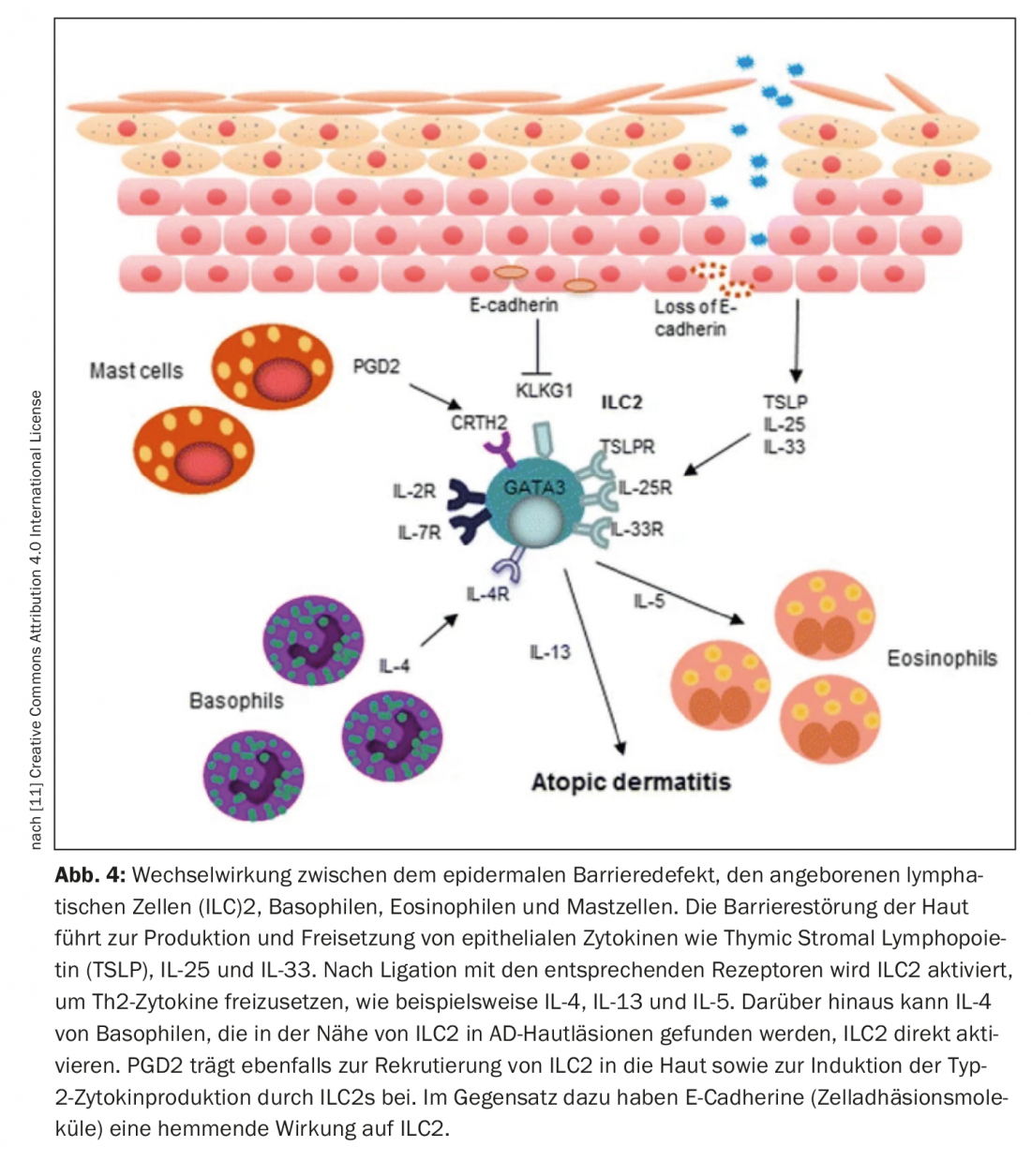
The pathogenesis of atopic dermatitis is based on a genetic skin barrier defect, dysbiosis of the cutaneous microbiome, and a Th2-dominated immune response. “Today we know that it is a multifactorial disease,” said Prof. Dr. med. Petra Staubach-Renz, Department of Dermatology and Polyclinic of the University Medical Center Mainz, on the occasion of this year’s congress Dermatology compact and practical. The cytokines interleukin(IL)-4 and IL-13 secreted by Th2 cells play a key role in the disease process of atopic dermatitis, especially in acute phases, explains Prof. Dr. med. Esther von Stebut-Borschitz, Clinic and Polyclinic for Dermatology and Venerology, University Hospital Cologne [1]. Overexpression of IL-13 has been found in both lesional and non-lesional atopic skin and, moreover, IL-13 levels have been found to correlate with the severity of atopic dermatitis [6]. One therapeutic strategy to inhibit biological activity is monoclonal antibodies that inhibit signaling through IL-13, for example, by blocking its binding to the receptor [2,3]. Tralokinumab is a biologic that specifically neutralizes IL-13 by binding to this cytokine, preventing its interaction with the IL-13Rα1 receptor [4]. “Tralokinumab blocks IL-13 so that the IL-13-R-α chain is not triggered,” summarized Prof. von Stebut-Borschitz [1].
Improvement of skin appearance and reduction of steroid use.
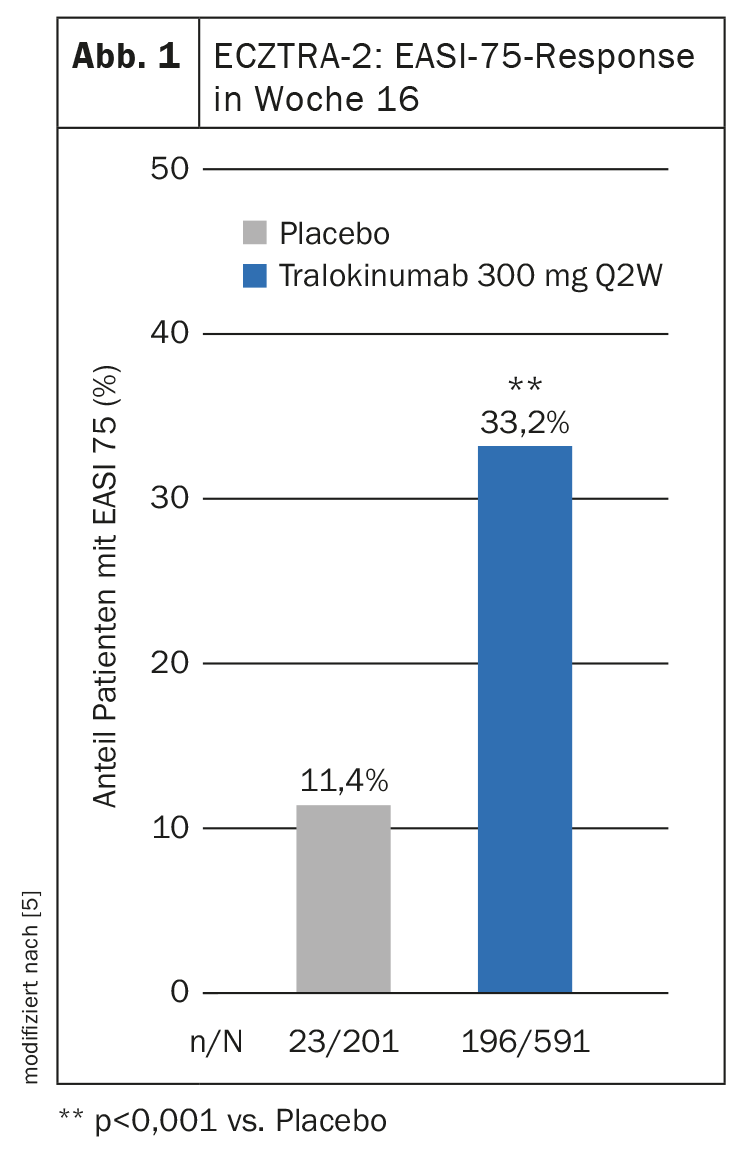
Two multinational phase III RCTs, ECZTRA-1 and -2, evaluated the efficacy and safety of 52 weeks of tralokinumab therapy in atopic dermatitis [5]. In both studies, tralokinumab 300 mg every two weeks (q2w) proved significantly superior to placebo in terms of improvements in the primary endpoints IGA 0/1 and EASI-75 at 16 weeks. The results of ECZTRA-2 show that after 16 weeks in the tralokinumab arm, a proportion of 33.2% achieved an EASI-75 (Fig. 1) [5]. In the placebo condition, this rate was 11.4%. In addition, steroid use was found to be lower in patients treated with tralokinumab compared with placebo.
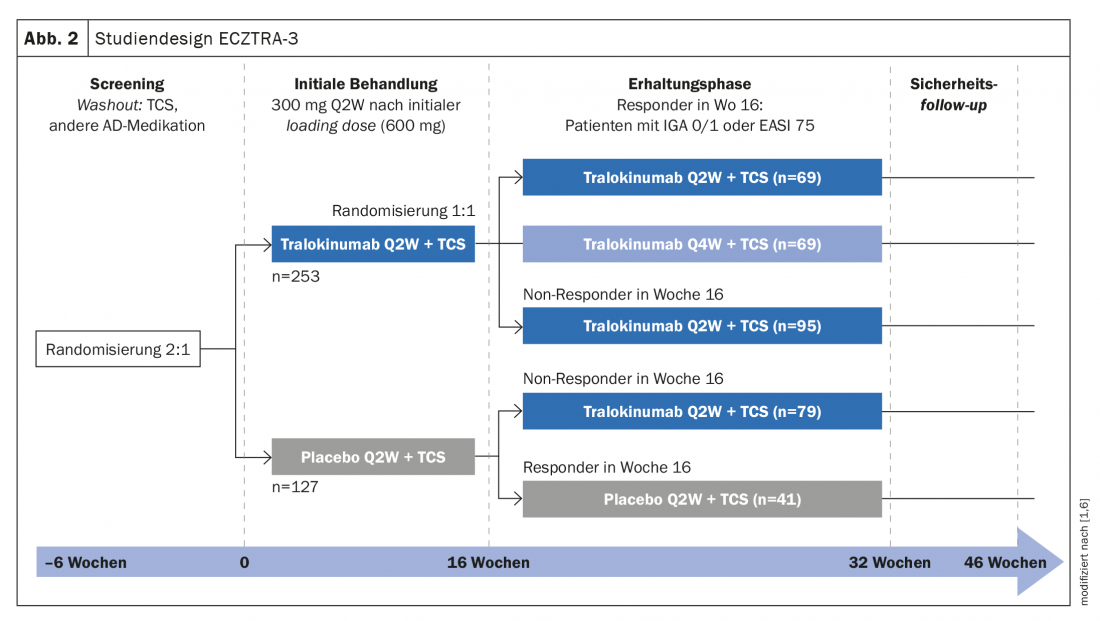
In ECZTRA-3, another phase III study, the efficacy and safety of tralokinumab 300 mg q2w plus topical corticosteroid (TCS*) were compared with placebo plus TCS [6]. The study population included 380 patients with moderate to severe atopic dermatitis. EASI-75 reached 56.0% in the tralokinumab arm vs. 5.7% in the placebo arm at week 16 (p<0.001). Regarding IGA 0/1, the corresponding values were 38.9% and 26.2%, respectively (p=0.015) [6]. Patients in the tralokinumab group showed reduced steroid use compared with the control group, emphasized Prof. von Stebut-Borschitz [1].
* On-demand therapy with locally applied cortisone was mometasone furoate 0.1% cream (class III steroid), applicable once daily to active lesions.
Patience pays off: subgroup showed delayed response
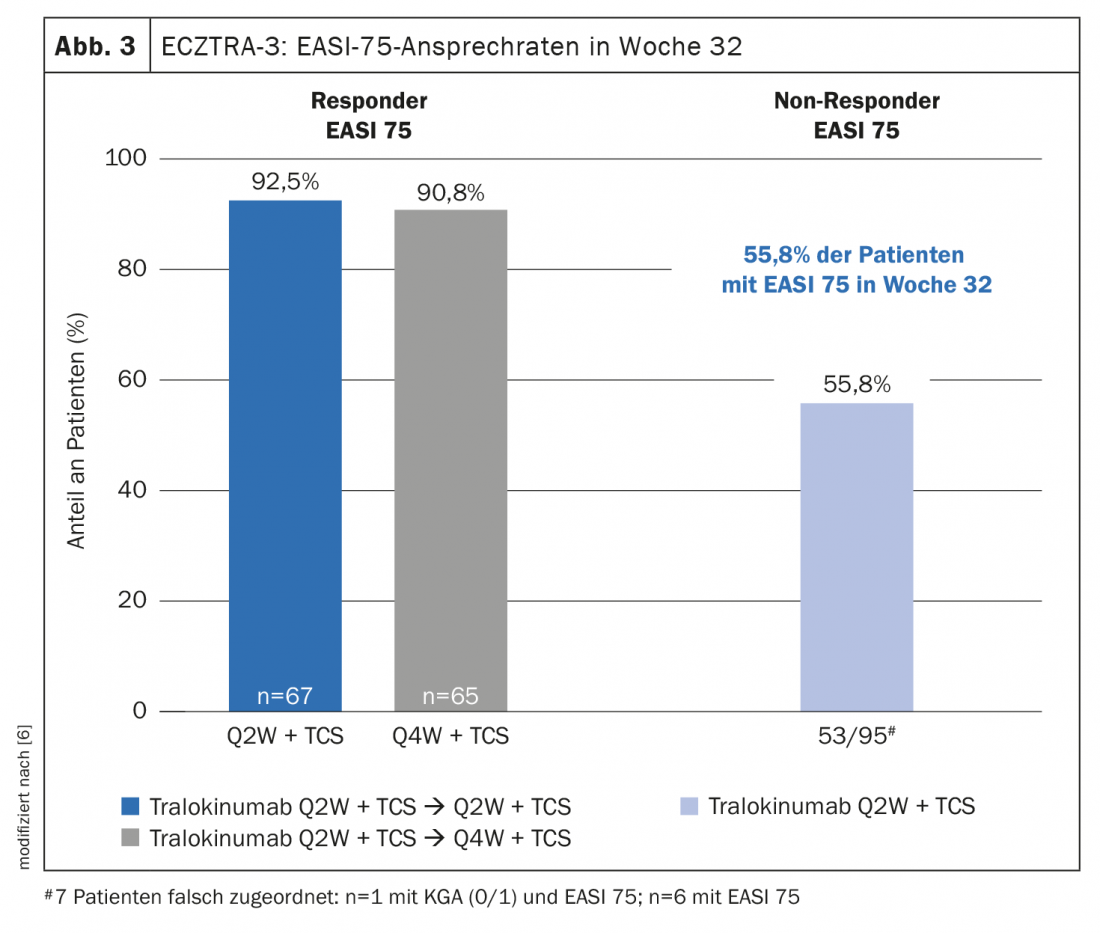
In the maintenance phase of the ECZTRA-3 trial, patients were re-randomized and data were analyzed for responders and non-responders. IGA 0/1 and EASI-75 at week 32 , of those patients who were responders at week 16, 89.6% and 92.5%, respectively, achieved q2w dosing regimen, whereas this proportion was 77.6% and 90.8%, respectively, in q4w dosing regimen [6]. With regard to long-term treatment with tralokinumab, it is interesting to note that even patients who do not respond sufficiently at first can develop a good response in the further course, explains Prof. von Stebut-Borschitz. In the non-responder subpopulation, that is, when neither IGA 0/1 nor EASI-75 had been achieved under tralokinumab q2w at week 16, a proportion of 30.5% and 55.8%, respectively, achieved these endpoints at week 32 under the q2w dosing regimen. “This is potentially very exciting for long-term management,” said the clinic director from Cologne [1]. It is also interesting to note that under q2w, the frequency of those reaching EASI-75 increases. “This means that delayed response is also possible, suggesting that we need to be a bit patient in starting therapy,” explained Prof. von Stebut-Borschitz [1]. In addition to IGA 0/1 and EASI-75, ECZTRA-3 data also demonstrated a benefit of tralokinumab in terms of itch relief [1]. “Already after 4 weeks you can see a strong reduction in itching, but this increases over time,” the speaker emphasized [1]. Moreover, quality of life improved significantly in patients treated with tralokinumab, as evidenced by the reduction in DLQI scores over the course of treatment [1]. Patients also benefited from therapy with tralokinumab with regard to sleep disturbances and bacterial colonization.
No loss of efficacy even after interruption of treatment
The objective of the phase III ECZTEND study is to investigate the long-term efficacy and safety of tralokinumab in patients with atopic dermatitis who had participated in previous studies [7]. Included in the interim analysis were data from subjects (n=345) who had received two years of treatment with tralokinumab, including the entire 52 weeks in the pivotal phase III main studies (ECZTRA-1 and -2) and 56 weeks in the ECZTEND study [8]. Subjects were divided into three cohorts based on the time interval between their last dose of tralokinumab in the main study and their first dose in ECZTEND. Continuous treatment was defined as ≤5 weeks between the last dose in the main study and the first dose in the ECZTEND study (n=126), a period of 6-15 weeks without tralokinumab was defined as treatment interruption (n=133), and >15 weeks (n=86) was assessed as treatment washout. From the analyses, interruption of tralokinumab therapy resulted in a drop in EASI scores, but after a renewed treatment period of two years, a total of 92.7% achieved a significant improvement in EASI compared with baseline in the respective main study. “Impressive long-term results showing that there is no loss of efficacy, even if a break is taken,” summarizes Prof. von Stebut-Borschitz [1].
Conjunctivitis: well controllable possible side effect
Regarding side effects, as with dupilumab, conjunctivitis is among the occasional adverse effects of treatment. Prof. von Stebut-Borschitz commented: “There are now very good therapy recommendations for the treatment of conjunctivitis, so that therapy only has to be stopped in rare cases” [1]. Ocular inflammation has been found to respond well to topical treatment [9]. This includes the use of moisturizing eye drops based on hyaluronic acid or with ingredients such as carbomer, hypromellose, dexpanthenol or povidone. In the eyelid edge area and when blepharitis also affects the eyelid, Tacrolimus ointment can be used. In patients who are prone to dry eyes or allergic conjunctivitis, preventive use of hyaluronic acid-containing eye drops or other tear substitutes may also be considered [9,10].
Congress: Dermatology compact and practical
Literature:
- “Beyond skin: tralokinumab and the long-term perspective for AD patients,” Lunch Symposium 06, Leo Pharma, Dermatology Compact and Practical Feb. 18-20, 2022.
- Bieber T: Allergy 2020; 75: 54-62.
- Schmid-Grendelmeier P: Atopic dermatitis: the role of the key cytokine IL-13. Dermatologie Praxis 2021; 31(4): 10-14.
- Wollenberg A, et al: J Dtsch Dermatol Ges 2021; 19(10): 1435-1442.
- Wollenberg A, et al: Br J Dermatol 2021; 184: 437-449.
- Silverberg JI, et al: Br J Dermatol 2021; 184: 450-463.
- Blauvelt A, et al: Two-year Maintenance of Response with Tralokinumab in Moderate-to-Severe Atopic Dermatitis: Interim Analysis of the ECZTEND Open-label Extension Trial. European Academy of Dermatology and Venereology (EADV), Sept 29-Oct 2, 2021. on-demand video oral presentation FC01.04.
- “LEO Pharma presents interim data from phase 3 tralokinumab long-term extension trial in moderate-to-severe atopic dermatitis at EADV 30th Congress,” Leo Pharma, Sept. 30, 2022.
- “Stellungnahme der GD Gesellschaft für Dermopharmazie e. V. Therapie der atopischen Dermatitis mit Biologika – Empfehlungen für die Patientenberatung in Klinik, Praxis und Apotheke”, 26.08.2021, http://gd-online.de, (last retrieval 22.03.2022).
- Aszodi N, et al: J Dtsch Dermatol Ges 2019; 17: 488-491.
- Rerknimitr P, et al: The etiopathogenesis of atopic dermatitis: barrier disruption, immunological derangement, and pruritus. Inflammation and Regeneration 2017; 37: 14, https://inflammregen.biomedcentral.com/articles (last accessed 23 Mar 2022).
DERMATOLOGIE PRAXIS 2022; 32(2): 20-23 (published 2/20/22, ahead of print).