Deep vein thrombosis develops in 1/1000 inhabitants of Western Europe per year. In the acute phase, it is critical to prevent the complication of pulmonary artery embolism. This is now effectively done at diagnosis with anticoagulation. Nevertheless, several thousand people die each year from this pulmonary thromboembolism because the diagnosis was not made.
Deep vein thrombosis develops in 1/1000 inhabitants of Western Europe per year [1]. In the acute phase, it is critical to prevent the complication of pulmonary artery embolism. This is now effectively done at diagnosis with anticoagulation. Nevertheless, about 25,000 people in Germany die of this pulmonary thromboembolism every year because patients with acute pulmonary artery embolism, among other causes, die suddenly or the diagnosis is made too late.
As effective as anticoagulation is in preventing thromboembolism, it is ineffective in preventing postthrombotic syndrome (PTS). In the long-term course, patients with deep vein thrombosis develop postthrombotic syndrome in 20-83% depending on the location and extent of the thrombosis [2,3]. The iliac veins are involved in iliofemoral thrombosis in about 40% of cases, and despite anticoagulation, recanalization is unsuccessful in 70% of cases [4,5]. This then leads to a chronic outflow obstruction and due to this to the PTS.
PTS summarizes the symptoms due to damaged and/or occluded deep veins caused by deep vein thrombosis. These generally include a feeling of heaviness, paresthesias, itching, cramps, pain, and peripheral edema in the legs. Skin indurations, hyperpigmentation, redness, venous ectasia, ulceration, and calf compression pain are also evident. Specifically, venous claudication is a leading symptom, leading to walking distance limitation similar to intermittent claudication. The ulcer cruris as the final stage of the symptomatology causes partly high costs for the health care system due to socioeconomic consequences such as reduced mobility and need for care [6,7].
Deep vein thrombosis
Diagnosis: In cases of suspected deep vein thrombosis, the diagnostic procedure is clearly defined in the guidelines. Any suspicion of deep vein thrombosis must be immediately investigated in order to initiate anticoagulant therapy if necessary. This is the only way to reduce the risk of pulmonary artery embolism and halt the progression of DVT [8]. The workup initially includes a medical history and physical examination. Due to the low sensitivity, this alone is not sufficient to diagnose or exclude thrombosis. Therefore, depending on the clinical probability (KW), laboratory diagnostics, especially determination of D-dimers, and/or compression ultrasound follow. Validated scores such as the commonly used Wells score [8] exist to determine KW for the presence of DVT.
Using the score, factors such as tumor disease or leg circumference difference are queried and assigned a score of 0 or 1. Based on this, a high or low KW for the presence of DVT is obtained. This should be documented as a separate diagnostic step. If KW is low, the D-dimer test usually follows. If this is negative, it is highly probable that DVT has been ruled out and no further diagnostic tests need to be performed [8]. If clinical probability is high, compression ultrasound follows. If this is inconclusive, further imaging or a repeat ultrasound examination should be performed after 3-5 days. It is recommended to examine the whole affected leg including pelvic veins with the help of compression ultrasound. If diagnostic imaging is not available in a timely manner, anticoagulation is indicated when clinical probability is high after weighing the risk of bleeding [8].
Therapy: Treatment of DVT includes anticoagulation, compression therapy, and rapid mobilization. It is also recommended to stop or switch the possible triggering risk factor such as contraception as well as to treat an underlying newly diagnosed disease such as tumor disease. Anticoagulation has been expanded in recent years with the New Oral Anticoagulants (NOAKs), which has certainly made it easier for patients to manage. However, NOAKs are increasingly interacting with other drugs such as antibiotics and antiarrhythmics. This should be checked at the time of preparation and during progress checks.
After maintenance therapy for 3-6 months, extended maintenance therapy should be discussed. The German Society of Angiology has developed a suitable traffic light scheme to help decide whether to continue therapy. (Table 1). It should be mentioned that the two studies with rivaroxaban and apixaban for prolonged maintenance therapy with lower doses have not yet received attention here, and these are also only approved for 1 year. [9,10]. Thrombophilia workup may be performed in selected patients during the course, but rarely has a therapeutic consequence. Examples of a therapeutic consequence have included antiphospholipid syndrome, antithrombin III deficiency, or significant inhibitor deficiency.

Clinical follow-up of the patient with DVT should be performed in the first 21 days, and sonographic follow-up should be performed after 3 months or after completion of anticoagulation.
For compression therapy, despite all the weaknesses in the study protocol, the Sox Trial showed no advantage for compression therapy in preventing post-thrombotic syndrome (PTS) [13]. However, compression is indispensable to relieve the symptoms; especially in the first weeks, compression therapy should be performed.
DVT of unclear etiology has the highest recurrence rate of up to 9% in the first year. The debate on whether DVT patients are at increased risk for tumor disease has not been conclusively decided [14,15]. If screening is performed, age-appropriate screening plus history and laboratory appears to be sufficient [16]. In younger patients, a vasculitis workup may be useful.
Tumor patients have varying risk of DVT depending on tumor entity and location. Patients with a pancreatic tumor have the highest risk of DVT. The occurrence of DVT is associated with a significantly worse outcome in tumor patients [17]. Tumor patients have a higher clinical likelihood from the outset and D-dimers are regularly elevated per se. Thus, clarification of possible DVT in tumor patients is recommended primarily with the aid of color duplex sonography and, if necessary, further imaging [18]. Tumor patients should be treated with a low molecular weight heparin for 3-6 months as this is superior to unfractionated heparin [19,20] and Marcumar [21–23]. Dosage and duration are to be made in consultation with the treating oncological colleagues. Due to the very high risk of recurrence of up to 20%, long-term anticoagulation is recommended depending on the risk of bleeding, at least as long as the tumor disease is present, even though the data in this regard are scarce.
Compared with low-molecular-weight heparin, several studies have been performed with the NOAKs in tumor patients. For edoxaban compared with dalteparin, there was a reduced recurrence rate (7.9% vs. 11.3%) but at the expense of an increased bleeding rate (6.9% vs. 4.0%) at 12 months [24]. The increased bleeding rate occurred mainly in the gastrointestinal tract in patients with gastrointestinal tumors. The Select-D pilot study evaluated rivaroxaban compared with dalteparin over a 6-month period. Again, rivaroxaban showed an advantage in the rate of recurrent thrombosis (4% vs. 11%), whereas the rate of major bleeding was increased (6% vs. 4%) [25]. In this study, the increased gastrointestinal bleeding rate was also notable. After the guidelines were updated in this regard, data on apixaban were still pending. An international randomized trial demonstrated noninferiority of apixaban to dalteparin in terms of recurrence rate (5.6% vs. 7.9%). Apixaban, however, unlike edoxaban and rivaroxaban, did not increase bleeding complications (3.8% vs. 4.0%) [26] in a comparable patient population.
In summary, NOAKs seem to be an alternative for tumor patients, but patients with gastrointestinal or urogenital tumors should currently be treated with great caution. NOAKs could be used from the diagnosis of DVT according to these data, but this depends on tumor entity, tumor stage, and current tumor treatment.
Lower leg thrombosis (distal deep vein thrombosis), like muscle vein thrombosis, is anticoagulated for a maximum of 3 months because of its prognostically favorable course, even in cases of recurrence or idiopathic genesis [27,28]. However, clear evidence for the necessity of anticoagulation is lacking, and the dosage is also currently under discussion.
|
LEFt score
|
One of the most common causes of death during pregnancy and puerperium is DVT, with a fourfold increased risk [29]. DVT occurs on the left leg in 70-90% of cases in pregnant women [30,31]. Pelvic vein thrombosis is also diagnosed more frequently during pregnancy. Therefore, the LEFt score with a high negative predictive value is used for diagnosis on the one hand, but needs further evaluation in a larger patient collective ( box) . D-dimers are elevated in pregnancy, but on the basis of Morse et al. and Chan et al. this can be adapted to the gestational age [32,33]. However, recent studies indicate, outside of high-risk patients, poor clinical practicality with use of D-dimers alone to exclude DVT [34]. Rather, clinical predictability rules appear useful in combination with D-dimer testing to save radiation in suspected pulmonary embolism.

In this regard, the pregnancy-adapted YEARS algorithm proposed in the current ESC guidelines for pulmonary embolism should not go unmentioned [35,36]. Finally, however, compression ultrasound should be used regularly to exclude DVT in pregnancy, if necessary also in the lateral position. If no conclusion about DVT can be made here, imaging by MR phlebography [37] avoiding gadolinium and using non-contrast enhanced sequences is recommended [8]. If compression ultrasound is negative, a checkup 7 days later is recommended [8].
If DVT is detected, anticoagulation with heparins, especially preferably low-molecular-weight heparin, is recommended and should be performed for 3 months at a therapeutic dose. Thereafter, a reduction in dose to intermediate (2× prophylactic) or prophylactic may be considered [8]. However, anticoagulation should be continued for at least 6 weeks postpartum [38]. NOAKs are contraindicated in pregnancy because of their placental toxicity, but also in the lactation period due to lack of safety data [39].
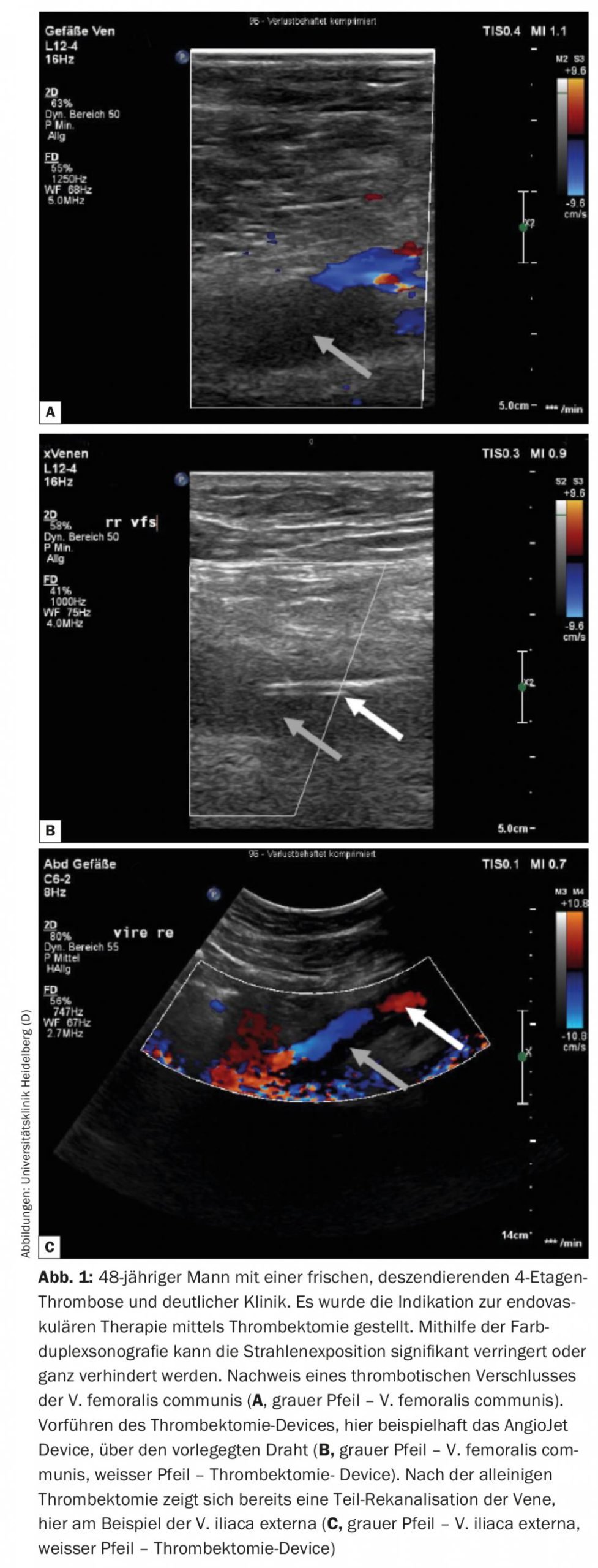
In patients with pronounced symptoms due to acute descending iliac vein thrombosis, an endovascular approach with low bleeding risk should be discussed. Descending thrombosis usually originates from a stenosis/occlusion in the inferior vena cava and/or iliac vein, resulting in rapid descending growth of the thrombus, leading to rapid intense symptomatology in the affected patient. Patients with this thrombosis entity, among others, benefit from an interventional approach according to a subgroup analysis of the Attract Trial [11]. Since these are mostly young patients, radiation exposure should be minimized or bypassed with the help of ultrasound-guided endovascular therapy (Fig. 1). Under ultrasound guidance, thrombectomy, local lysis therapy, and PTA and stent implantation can be performed, as partially shown in the following case report [12].
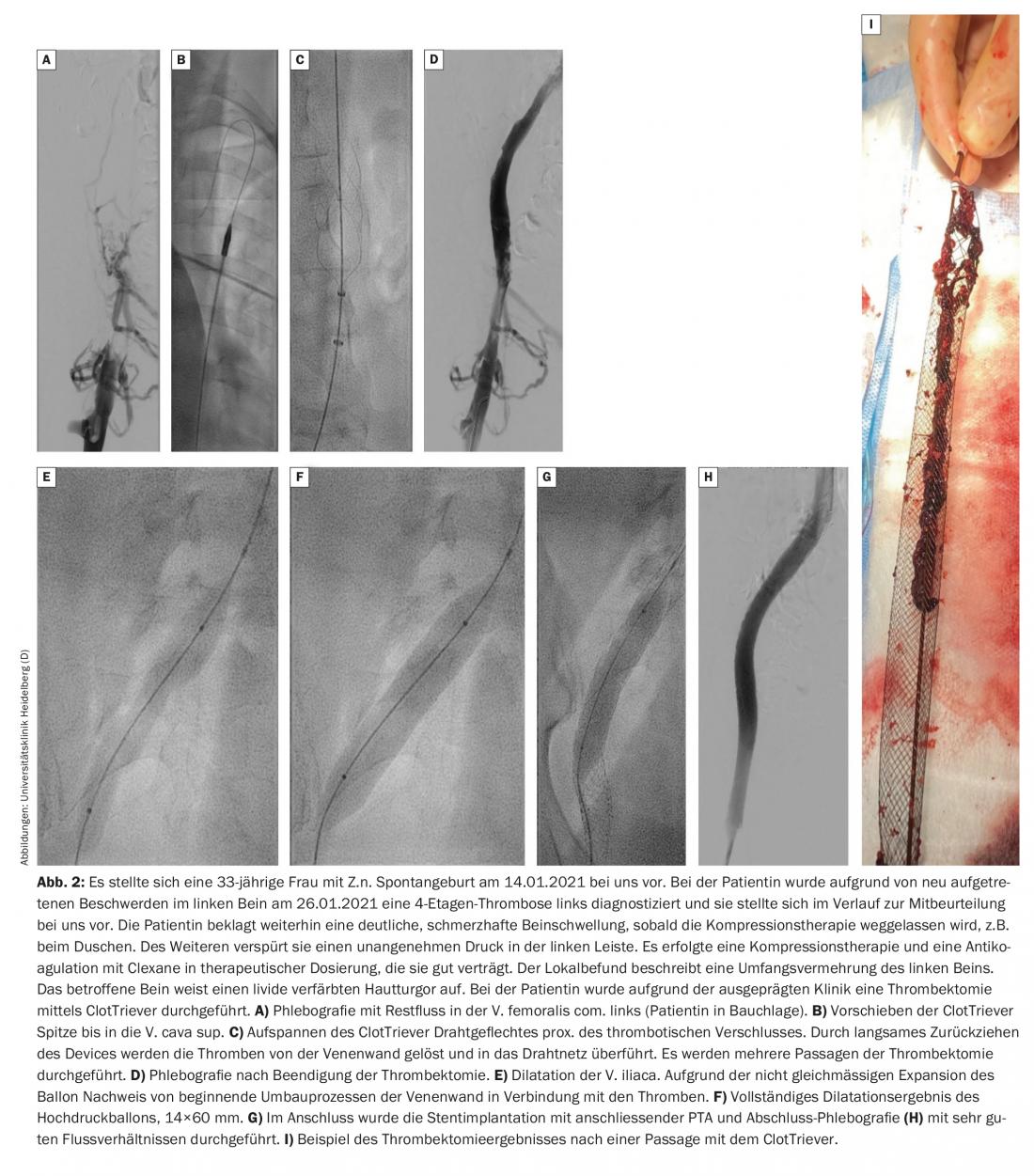
According to the Heidelberg protocol, patients are initially treated conservatively for a few days. If there is no significant improvement in symptoms or thrombus extension during this period, an endovascular procedure should be discussed with the patient and performed if necessary. Thrombectomy should be performed within 14 days of symptom onset if possible. A new device, the so-called ClotTriever, apparently also makes it possible to effectively treat subacute iliac vein thrombosis. A registry is currently underway and it remains to be seen what the initial results will be (Fig. 2).
Therapy for postthrombotic syndrome (PTS)
Indication and diagnostic work-up before endovascular surgery: The indication for endovascular surgery depends on the patient’s symptoms. From CEAP stage 3, there is an indication for reopening of the occluded veins according to the American recommendations. The following veins can be treated with the help of endovascular interventions: V. femoralis communis, V. iliaca, V. cava inferior and superior and partly the V. subclavia. Prior to intervention, diagnostic testing is first performed with clinical examination, a walk test, color duplex sonography, and additional imaging such as an MR or CT phlebotomy, if necessary. The extent of vein occlusion as well as inflow and outflow are essential here, as the long-term openness rate depends on these factors.
Therapy: The basic therapy of PTS consists of compression therapy, physical measures and exercise. Exercise is essential for the formation of bypass circuits and thus the improvement of symptomatology. In addition, optimal wound treatment is elementary in the case of a leg ulcer.
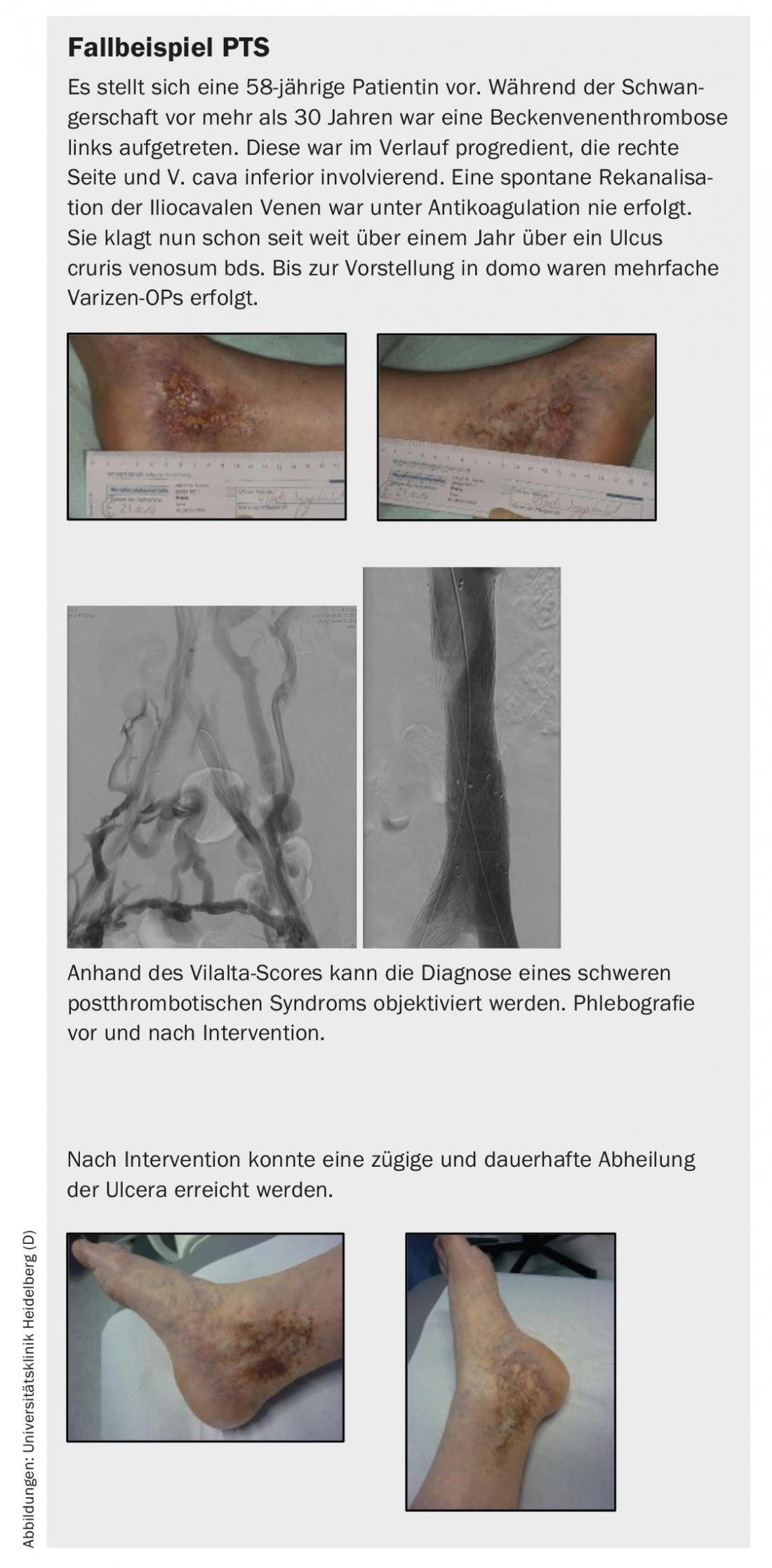
Invasive treatment of proximal venous thrombosis or chronic venous occlusion was introduced internationally 30 years ago; in our country, endovascular interventions have been successfully performed in major centers for several years with high openness rates and result in significant improvement of patient symptoms and healing of venous ulcers.
If the clinic is appropriate, a possible venous intervention can be discussed with the patient and the advantages as well as disadvantages or risks can be discussed. It does not matter how old the occlusion is in terms of the chances of success of the procedure. The primary open rate is almost 70% after 5 years. In this meta-analysis, there was also a significant improvement in the symptoms of discomfort; freedom from pain was achieved in up to 82% of the treated patients after 5 years. In addition, the procedure resulted in long-term healing of a crural ulcer in up to 80% [1].
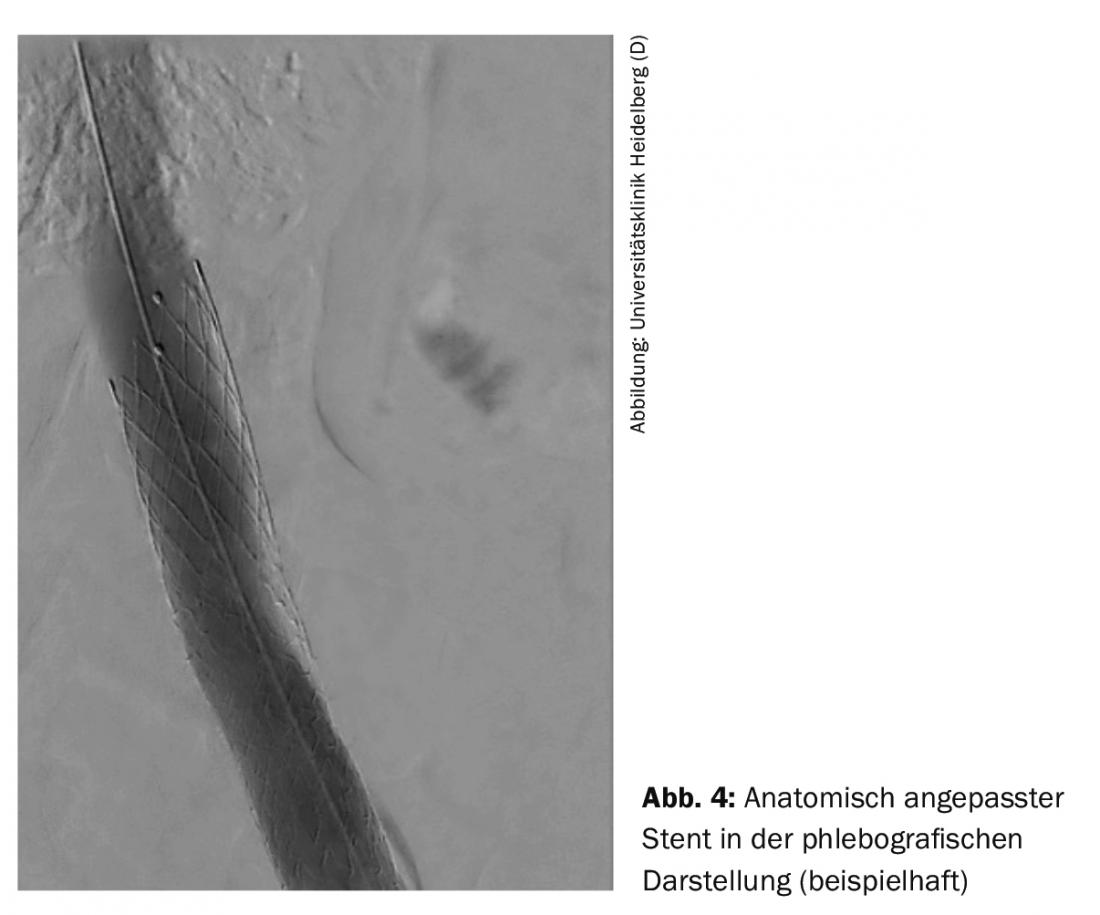
The endovascular procedure is usually performed via the popliteal vein. Other access routes include the femoral vein and the jugular vein. Depending on the case, the procedure can be performed under analgosedation or intubation anesthesia. After phlebography, wire passage through the occlusion takes place. Subsequently, due to the rigidity of the venous occlusions, special high-pressure balloons with diameters of 14-16 mm in the iliac vein and 20-24 mm in the inferior vena cava are used for adequate dilatation. used. After dilation, the vein wall is not a stable construct and collapses rapidly, so stents designed specifically for the veins are used to ensure a long-term open rate. Anatomically adapted stents are available to achieve optimal stent placement during unilateral intervention in the proximal iliac vein (Fig. 4).
Other patient collectives: Complementary etiologies include compression of the vein by arteries, so-called non-thrombotic venous stenosis (NIVL), or space-occupying lesions such as tumors. NIVL usually involves compression of the iliac vein com. left through the A. iliaca com. right, the so-called May-Thurner stenosis. If complaints are added, then one speaks of a May-Thurner syndrome. These NIVL compressions can be effectively performed today with a very high openness rate of approximately 90% at 5 years with short intervention time and low area dose product [1].
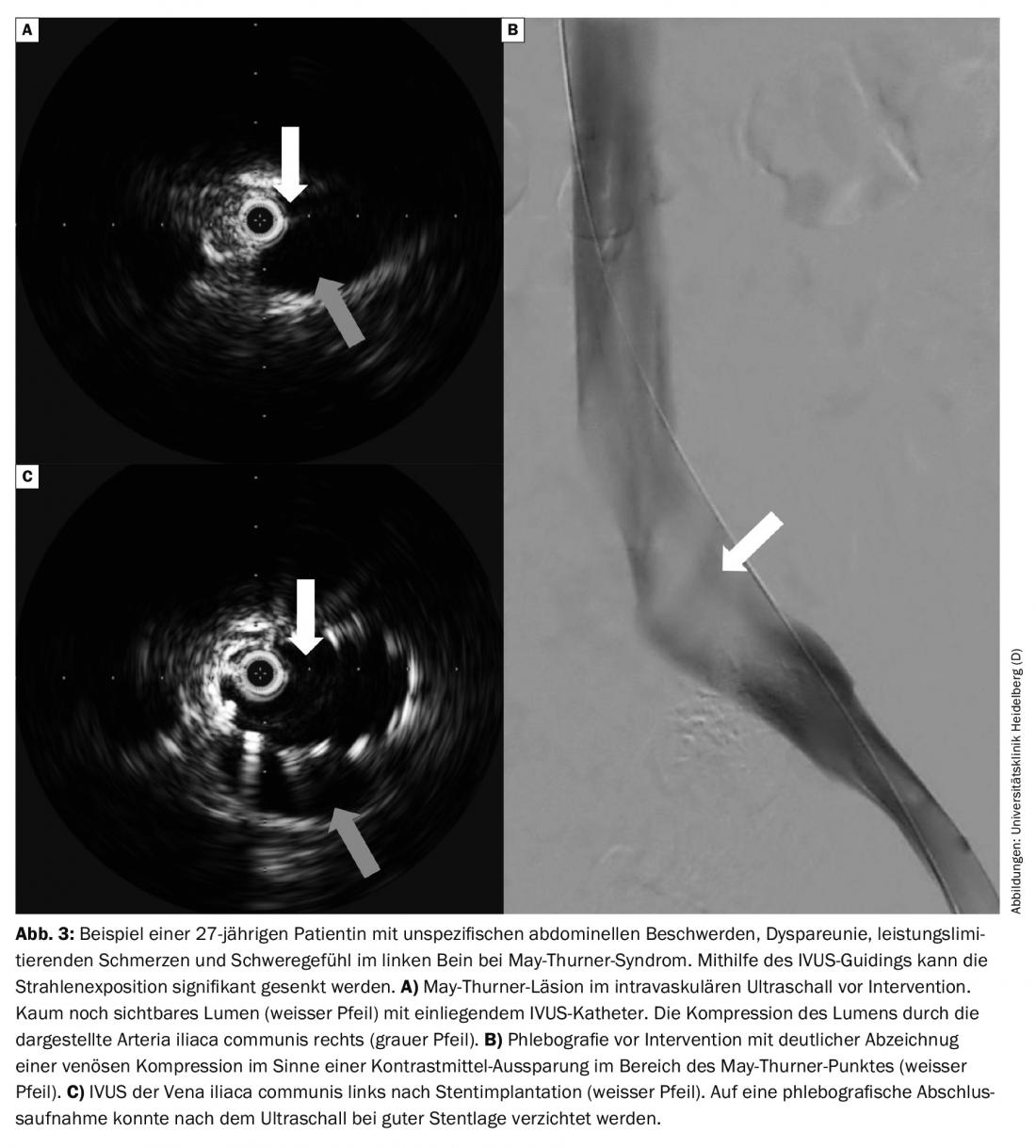
The limitations of color duplex sonography, CT, and MR phlebography in detecting iliac dynamic obstruction can now be met by the use of intravascular ultrasound (IVUS) when pelvic vein thrombosis is suspected with typical symptoms without diagnostic correlates (Fig. 3A). In this way, X-ray doses can be saved or even dispensed with in the diagnosis of this often younger group of patients. If intervention is necessary, IVUS can be an aid in choosing stent size and length.
Invasive treatment of venous compression by a tumor can lead to rapid symptom relief, not only in the palliative setting. After stent placement and post-dilatation, the stent reaches its optimal shape to keep the corresponding vein open with outward forces.
Follow-up: Follow-up visits are recommended at 1,3 and 12 months. Clinical and color duplex sonographic follow-up is performed on these days. Repeat phlebography, or CTA, is not routinely necessary.
Postinterventional antithrombotic treatment depends on the etiology of the individual’s risk of thrombosis and bleeding. It is done individually. In most cases, therapeutic full anticoagulation is necessary for 6-12 months. Subsequently, the further procedure is discussed and carried out individually with the patient. In addition, platelet inhibition may be advisable for the first 6 months. NIVL require a less tight regimen than complex chronic occlusions following intervention. Full anticoagulation for 3-6 months is recommended. Anticoagulation is then discontinued after this.
In summary, endovascular therapy of venous occlusions is a very good way to effectively help patients with more significant symptoms in the long term.
Take-Home Messages
- NOAK therapy may be used in tumor patients.
- However, this depends on the tumor entity (not GI and genitourinary tumors), the tumor stage and their therapy.
- Guideline-based anticoagulation minimizes the rate of thromboembolism, but not the likelihood of occurrence of postthrombotic syndrome (PTS).
- In acute, clearly symptomatic pelvic vein thrombosis, interventional procedures should be discussed if symptoms and thrombus extension fail to improve with conservative therapy.
- In postthrombotic syndrome, endovascular reopening of occluded veins can significantly improve quality of life in symptomatic patients. In addition, this is an effective therapy for long-term healing of venous ulcers.
- In nonthrombotic venous stenoses (e.g., May-Thurner stenosis), the openness rate after intervention is approximately 90% at 5 years.
Literature:
- Razavi MK, Jaff MR, Miller LE: Safety and Effectiveness of Stent Placement for Iliofemoral Venous Outflow Obstruction: Systematic Review and Meta-Analysis. Circ Cardiovasc Interv 2015; 8: e002772.
- Kahn SR, Shrier I, Julian JA, et al: Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med 2008; 149: 698-707.
- Prandoni P, Lensing AW, Cogo A, et al: The long-term clinical course of acute deep venous thrombosis. Ann Intern Med 1996; 125: 1-7.
- Johnson BF, Manzo RA, Bergelin RO, Strandness DE, Jr: Relationship between changes in the deep venous system and the development of the postthrombotic syndrome after an acute episode of lower limb deep vein thrombosis: a one- to six-year follow-up. J Vasc Surg 1995; 21: 307-312; discussion 13.
- Akesson H, Brudin L, Dahlstrom JA, et al: Venous function assessed during a 5 year period after acute ilio-femoral venous thrombosis treated with anticoagulation. Eur J Vasc Surg 1990; 4: 43-48.
- Ashrani AA, Heit JA: Incidence and cost burden of post-thrombotic syndrome. J Thromb Thrombolysis 2009; 28: 465-476.
- Guanella R, Ducruet T, Johri M, et al: Economic burden and cost determinants of deep vein thrombosis during 2 years following diagnosis: a prospective evaluation. J Thromb Haemost 2011; 9: 2397-2405.
- S2 Guideline: Diagnosis and Therapy of Venous Thrombosis and Pulmonary Embolism. www.awmf.org/leitlinien/detail/ll/065-002.html. Current status: October 10, 2015.
- Agnelli G, Buller HR, Cohen A, et al: Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013; 368: 699-708.
- Weitz JI, Lensing AWA, Prins MH, et al: Rivaroxaban or Aspirin for Extended Treatment of Venous Thromboembolism. N Engl J Med 2017; 376: 1211-1222.
- Comerota AJ, Kearon C, Gu CS, et al: Endovascular Thrombus Removal for Acute Iliofemoral Deep Vein Thrombosis. Circulation 2019; 139: 1162-1173.
- Heckmann MB, Wangler S, Katus HA, Erbel C: Ultrasound-assisted endovascular treatment of acute venous thromboses. Vasa 2019; 48: 443-449.
- Kahn SR, Shapiro S, Wells PS, et al: Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet 2014; 383: 880-888.
- Carrier M, Lazo-Langner A, Shivakumar S, et al: Screening for Occult Cancer in Unprovoked Venous Thromboembolism. N Engl J Med 2015; 373: 697-704.
- Robin P, Le Roux PY, Planquette B, et al: Limited screening with versus without (18)F-fluorodeoxyglucose PET/CT for occult malignancy in unprovoked venous thromboembolism: an open-label randomised controlled trial. Lancet Oncol 2016; 17: 193-199.
- Khan F, Vaillancourt C, Carrier M.: Should we screen extensively for cancer after unprovoked venous thrombosis? BMJ 2017; 356: j1081.
- Levitan N, Dowlati A, Remick SC, et al: Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data. Medicine (Baltimore) 1999; 78: 285-291.
- Gary T: Cancer related venous thromboembolism – prophylaxis and therapy. Vasa 2014; 43: 245-251.
- Akl EA, Rohilla S, Barba M, et al: Anticoagulation for the initial treatment of venous thromboembolism in patients with cancer: a systematic review. Cancer 2008; 113: 1685-1694.
- Akl EA, Kahale L, Neumann I, et al: Anticoagulation for the initial treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev 2014: CD006649.
- Akl EA, Kahale L, Barba M, et al: Anticoagulation for the long-term treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev 2014: CD006650.
- Hull RD, Pineo GF, Brant RF, et al: Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med 2006; 119: 1062-1072.
- Lee AYY, Kamphuisen PW, Meyer G, et al: Tinzaparin vs Warfarin for Treatment of Acute Venous Thromboembolism in Patients With Active Cancer: A Randomized Clinical Trial. JAMA 2015; 314: 677-686.
- Raskob GE, van Es N, Verhamme P, et al: Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N Engl J Med 2018; 378: 615-624.
- Young AM, Marshall A, Thirlwall J, et al: Comparison of an Oral Factor Xa Inhibitor With Low Molecular Weight Heparin in Patients With Cancer With Venous Thromboembolism: Results of a Randomized Trial (SELECT-D). J Clin Oncol 2018; 36: 2017-2023.
- Agnelli G, Becattini C, Meyer G, et al: Apixaban for the Treatment of Venous Thromboembolism Associated with Cancer. N Engl J Med 2020; 382: 1599-1607.
- Galanaud JP, Sevestre MA, Genty C, et al: Incidence and predictors of venous thromboembolism recurrence after a first isolated distal deep vein thrombosis. J Thromb Haemost 2014; 12: 436-443.
- Baglin T, Douketis J, Tosetto A, et al: Does the clinical presentation and extent of venous thrombosis predict likelihood and type of recurrence? A patient-level meta-analysis. J Thromb Haemost 2010; 8: 2436-2442.
- Marik PE, Plante LA: Venous thromboembolic disease and pregnancy. N Engl J Med 2008; 359: 2025-2033.
- Chan WS, Spencer FA, Ginsberg JS: Anatomic distribution of deep vein thrombosis in pregnancy. CMAJ 2010; 182: 657-660.
- Ginsberg JS, Brill-Edwards P, Burrows RF, et al: Venous thrombosis during pregnancy: leg and trimester of presentation. Thromb Haemost 1992; 67: 519-520.
- Morse M: Establishing a normal range for D-dimer levels through pregnancy to aid in the diagnosis of pulmonary embolism and deep vein thrombosis. J Thromb Haemost 2004; 2: 1202-1204.
- Chan WS, Lee A, Spencer FA, et al: D-dimer testing in pregnant patients: toward determining the next ‘level’ in the diagnosis of deep vein thrombosis. J Thromb Haemost 2010; 8: 1004-1011.
- Goodacre S, Horspool K, Nelson-Piercy C, et al: The DiPEP study: an observational study of the diagnostic accuracy of clinical assessment, D-dimer and chest x-ray for suspected pulmonary embolism in pregnancy and postpartum. BJOG 2019; 126: 383-392.
- Konstantinides SV, Meyer G, Becattini C, et al: 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 2020; 41: 543-603.
- van der Pol LM, Tromeur C, Bistervels IM, et al: Pregnancy-Adapted YEARS Algorithm for Diagnosis of Suspected Pulmonary Embolism. N Engl J Med 2019; 380: 1139-1149.
- Tan M, Mol GC, van Rooden CJ, et al: Magnetic resonance direct thrombus imaging differentiates acute recurrent ipsilateral deep vein thrombosis from residual thrombosis. Blood 2014; 124: 623-627.
- Chan WS, Rey E, Kent NE, et al: Venous thromboembolism and antithrombotic therapy in pregnancy. J Obstet Gynaecol Can 2014; 36: 527-553.
- Cohen H, Arachchillage DR, Middeldorp S, et al: Management of direct oral anticoagulants in women of childbearing potential: guidance from the SSC of the ISTH. J Thromb Haemost 2016; 14: 1673-1676.
- Bauersachs, et al: Dtsch Med Wochenschr 2018; 143: 1-6 (13).
CARDIOVASC 2022; 21(1): 12-19