Treatment options for psoriasis patients in childhood and adolescence are much more limited compared to the treatment spectrum for adults. But pediatric patients can also benefit from targeted treatment. This is shown, among other things, by corresponding clinical study data on secukinumab. The long-term data from the extension phase of the pivotal studies were also convincing.
The EU approval of secukinumab in pediatric patients was based on the results of a phase III study program in which secukinumab led to rapid and durable healing of skin lesions in 6- to <18-year-old study participants with moderate to severe plaque psoriasis [1]. The biologic not only proved effective, but was generally well tolerated [2,3]. The safety profile corresponded to that in adult psoriasis patients.
| Targeted therapy for juvenile plaque psoriasis |
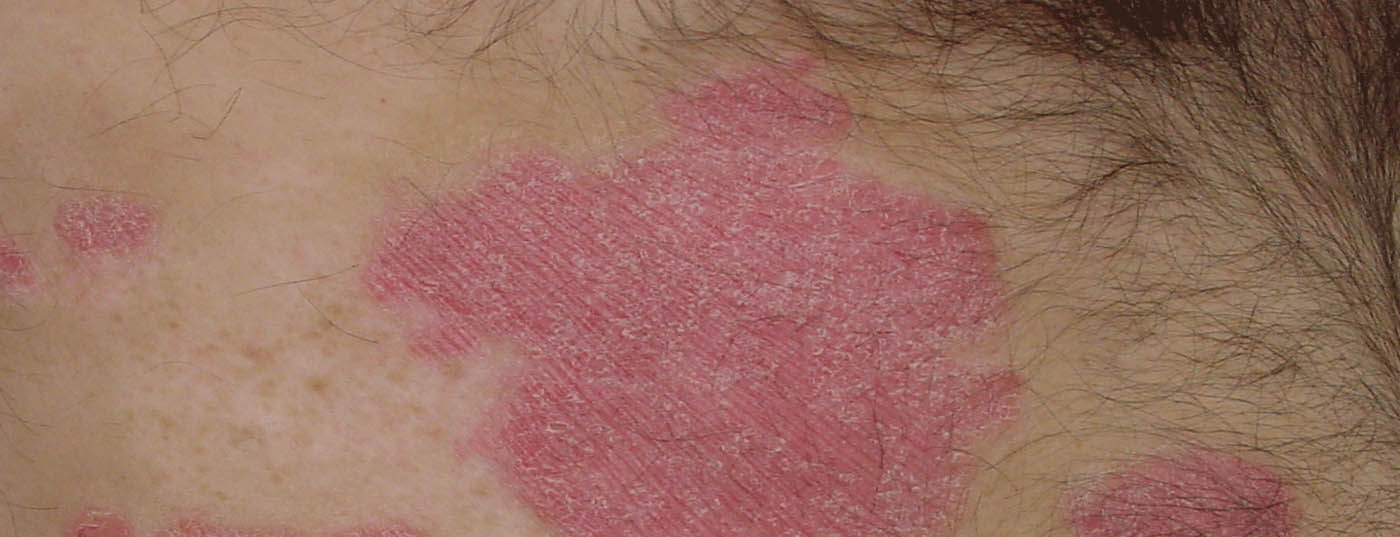
| Around 1% of children and adolescents are affected by plaque psoriasis [8–12]. Psoriasis is often associated with metabolic, cardiovascular, gastrointestinal and psychological comorbidities and a significantly impaired quality of life [8–12]. In pediatric patients, the impact of psoriasis on quality of life is thought to be similar to that of asthma or arthritis [7]. |
| While many effective and well-tolerated therapies are available for the treatment of psoriasis in adults, the treatment options for patients in childhood and adolescence are much more limited [8–12].
This is at least partly due to a lack of predictability of response to treatment options approved for adults [8]. Several targeted biologic agents have recently been approved in the EU for the treatment of pediatric plaque psoriasis, including secukinumab (Cosentyx®), an IL-17A antagonist whose pharmacological properties have been extensively studied [13]. IL-17 is an important proinflammatory cytokine involved in the pathogenesis of psoriasis, IL-17A is upregulated in lesional and non-lesional psoriatic skin [14]. |
Clinical response sustained over treatment period of 2 years
The first of the phase III studies included pediatric patients who had been diagnosed with severe chronic plaque psoriasis at least three months prior and were eligible for systemic therapy [2]. At baseline, study participants had a PASI* score of ≥20, an IGA# score of 4 and body surface area involvement (BSA**) of ≥10%. The mean age was 13.5 years, and most (77.2%) were ≥12 years old. The mean overall BSA was 40% and the mean duration of plaque psoriasis was 5.22 years. Comorbid psoriatic arthritis was present in 8.6% of patients [2]. Patients were randomized into different dosage groups stratified by body weight (bw): low dosage (LD): 75 mg for bw <50 kg and 150 mg for bw ≥50 kg, high dosage (HD): 75 mg for bw <25 kg, 150 mg for bw 25 to < 50 kg and 300 mg for bw ≥50 kg. In the comparator treatment arm, etanercept (s.c) 0.8 mg/kg bw (up to a maximum of 50 mg) was administered.
* PASI = Psoriasis Area Severity Index
# IGA = Investigator’s Global Assessment Modified 2011
** BSA = Body Surface Area
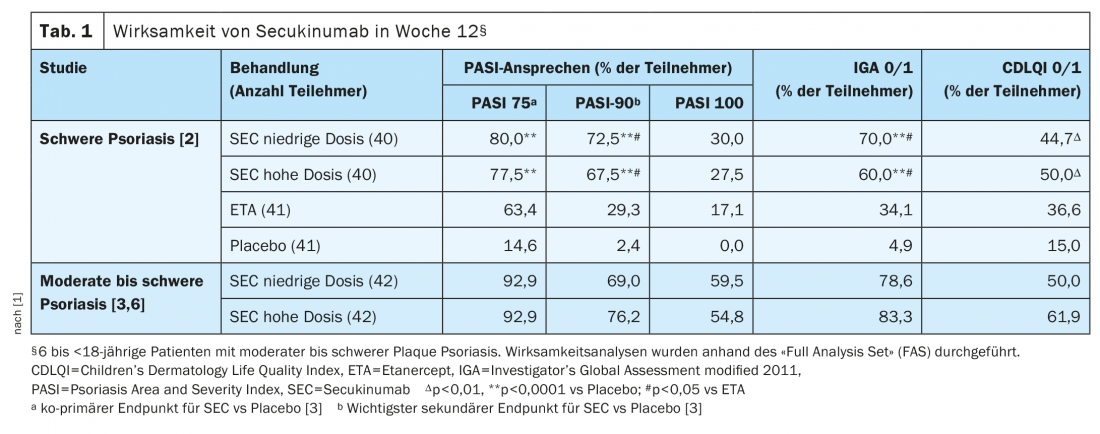
The clinical efficacy of secukinumab was already evident at week 4. At week 12, the PASI-75, IGA 0/1 and PASI-90 response rates were significantly higher with both low- and high-dose secukinumab than with placebo (Table 1). And compared to etanercept, secukinumab (LD and HD) showed significantly higher IGA 0/1 and PASI-90 response rates and numerically higher PASI-75 and PASI-100 response rates at week 12 (Tab. 1). In patients with a bw ≥25 to <50 kg, PASI-75/90/100 and IGA 0/1 response rates at week 12 were numerically higher with high-dose secukinumab (n=15) than in the lower-dose secukinumab groups (n=17) [2]. Over the same period, mean PASI scores improved from baseline by 82.9% in the low-dose secukinumab group and by 79.9% in the high-dose secukinumab group, compared with 29.3% in the placebo group and 74.2% under etanercept [2]. Both PASI response rates and IGA 0/1 response persisted under secukinumab until week 104. At week 52, the proportion with PASI-75 response was numerically higher at the high and lower doses compared to etanercept (87.5% and 87.5% vs. 68.3%).
IGA 0/1 response rates of over 80% at week 52.
The second of the randomized controlled pivotal clinical trials included patients aged 6 to <18 Jahren, die seit mindestens drei Monaten von einer mittelschwer bis schwer ausgeprägten Plaque-Psoriasis betroffen waren und für eine systemische Therapie geeignet waren [3]. Einschlusskriterien waren ein PASI-Score>12, IGA score >3 and BSA involvement >10%. At baseline, 72.6% of patients had moderate psoriasis and 27.4% had severe psoriasis. The average age of the patients was 12.6 years and 60.7% of the patients were between 12 and <18 years old. The average BSA participation was 30%. In this study, too, patients received low-dose (LD) or high-dose secukinumab (HD) depending on body weight and disease severity. In the LD subgroup, patients with a body weight (BW) <50 kg were treated with 75 mg secukinumab and those with a BW ≥50 kg with 150 mg secukinumab. In the HD group, patients with a bw <25 kg received a secukinumab dose of 75 mg, those with a bw between 25 to <50 kg were given a dose of 150 mg and patients with a bw ≥50 kg received 300 mg.
Secukinumab was administered at weeks 0, 1, 2, 3 and 4 and subsequently every 4 weeks. Placebo data from previous controlled trials in adult and pediatric patients with plaque psoriasis were used for the primary and main secondary endpoint analyses. The co-primary endpoint was the proportion of patients who achieved a PASI-75 response and an IGA 0/1 response at week 12 with secukinumab compared to placebo (documented from previous studies) [3].
At week 12, both low- and high-dose secukinumab were superior to the placebo response rates of PASI-75, IGA 0/1 and PASI-90 documented from previous studies. The estimated probability of a positive treatment effect was 1 (100%) [3]. PASI-75 and IGA 0/1 response rates increased until week 24 (LD groups: 95.2% and 88.1%; HD groups: 95.2% and 92.9%) and persisted until week 52 (LD: 88.1% and 85.7%; HD: 90.5% and 83.3%) [3,4]. The PASI 90/100 response rates at week 52 were 76.2/52%/52,4% in the low-dose secukinumab group and 83.3/69%/69,0% in the high-dose secukinumab group [4]. PASI90 response rates at weeks 32, 48, and 52 were numerically higher in the high-dose secukinumab groups compared with the lower-dose group. The PASI-100 response rates showed a similar pattern from week 32 to week 52. Overall, secukinumab proved to be effective in all subgroups regardless of body weight (<25 kg, 25 to <50 kg and ≥50 kg) and age (6 to <12 years and 12 to <18 years) [5].
| Cosentyx® for pediatric patients: Expansion of the therapeutic spectrum |
| Secukinumab (Cosentyx®) has been shown to be an effective treatment for children and adolescents with moderate to severe plaque psoriasis, with generally good tolerability. It is a valuable addition to the limited treatment options for this patient population and received EMA approval in 2020. |
| Targeted therapies with biologics offer several advantages over conventional systemic therapies, including less frequent dosing, improved efficacy and a reduced need for laboratory monitoring. |
| Like other biologics approved for pediatric psoriasis vulgaris, secukinumab is administered by subcutaneous injection.
Following appropriate instructions, Cosentyx® can be administered by an adult caregiver using a pre-filled syringe or pen. In contrast to etanercept, which is administered once a week, and adalimumab, which is administered every 2 weeks, the dose interval for secukinumab in maintenance therapy is 4 weeks. The less frequent administration and the possibility of home treatment are factors that can have a favorable effect on treatment adherence. |
| to [1,7] |
Safety profile comparable to that in adults
The tolerability profile of secukinumab in pediatric patients was consistent with safety data observed in adult psoriasis patients. Secukinumab was generally well tolerated, with nasopharyngitis being the most commonly reported adverse event (AE). Rates of AEs of special interest were generally low. No new safety signals were identified during the 52- and 104-week treatment periods of the extension study.
Literature:
- Blair HA: Secukinumab: A Review in Moderate to Severe Pediatric Plaque Psoriasis. Pediatric Drugs 2021; 23: 601-608.
- Bodemer C, et al: Secukinumab demonstrates high efficacy and a favorable safety profile in paediatric patients with severe chronic plaque psoriasis: 52-week results from a phase 3 double-blind randomized, controlled trial. J Eur Acad Dermatol Venereol 2020; 35(4): 938-947.
- Magnolo N, et al.: A phase III open-label, randomized multicenter study to evaluate efficacy and safety of secukinumab in pediatric patients with moderate to severe plaque psoriasis: 24-week results. J Am Acad Dermatol 2021; S0190-9622(21)02509-3
- Reich A, et al.: Secukinumab treatment demonstrated high efficacy and safety in paediatric patients with moderate-to-severe plaque psoriasis: 52-week results from a randomised trial [abstract no. P121 plus poster]. Pediatr Dermatol 2021; 38(Suppl. 1): 57–58.
- Szepietowski JC, et al.: Secukinumab demonstrated consistent efficacy across age and weight subgroups in pediatric patients with psoriasis: analyses from two phase 3 pediatric studies [abstract no. 78 plus poster]. In: Society for Pediatric Dermatology. 2021.
- Beissert S, et al. Secukinumab improves quality of life of paediatric patients with moderate-to-severe plaque psoriasis: 52-week results from a phase III, randomised study [abstract no. P080 plus poster]. Pediatr Dermatol. 2021;38(Suppl. 1): 43.
- Cordoro KM:. Toward optimal care of the pediatric patient with psoriasis: the new AAD-NPF management guideline. J Psoriasis Psoriatic Arthritis 2020; 5(1): 7–11.
- Nogueira M, Paller AS, Torres T: Targeted therapy for pediatric psoriasis. Paediatr Drugs 2021; 23(3): 203–212.
- Wu JJ, et al.: Treatment of psoriasis with secukinumab in challenging patient scenarios: a review of the available evidence. Dermatol Ther 2020; 10(3): 351–364.
- Menter A, et al: Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients. J Am Acad Dermatol 2020; 82(1): 161-201.
- Eisert L, et al: S2k guidelines for the treatment of psoriasis in children and adolescents – short version part 1. J Dtsch Dermatol Ges 2019; 17(8): 856-870.
- Haulrig MB, Zachariae C, Skov L: Off-label treatments for pediatric psoriasis: lessons for the clinic. Psoriasis (Auckl) 2021; 11: 1–20.
- Drug information, www.swissmedicinfo.ch,(last accessed 26.11.2021)
- Blauvelt A, Chiricozzi A: The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin Rev Allergy Immunol 2018; 55(3): 379–390.
DERMATOLOGIE PRAXIS 2021; 31(6): 28–29