In the context of anthropogenic climate change and global warming, questions are often raised about the resulting potential for tropical vectors (and the diseases they transmit) to spread due to rising temperatures. This is a very valid question, but not one that can be answered in a sweeping and simple manner. In addition, there are strong regional differences, although only Europe is considered here.
In the context of anthropogenic climate change and global warming, questions are often raised about the resulting potential for tropical vectors (and the diseases they transmit) to spread due to rising temperatures. This is a very valid question, but not one that can be answered in a sweeping and simple manner. In addition, there are strong regional differences, although only Europe is considered here.
Basically, the species composition in a region (e.g. a continent or part of it) is never static, but changes regularly in the course of evolution. However, these natural processes occur on much larger time scales than the human lifespan. This contributes to our subjective perception of the occurrence of certain animal and plant species in our environment as static, and to our labeling of species as “native” or “alien/introduced.”
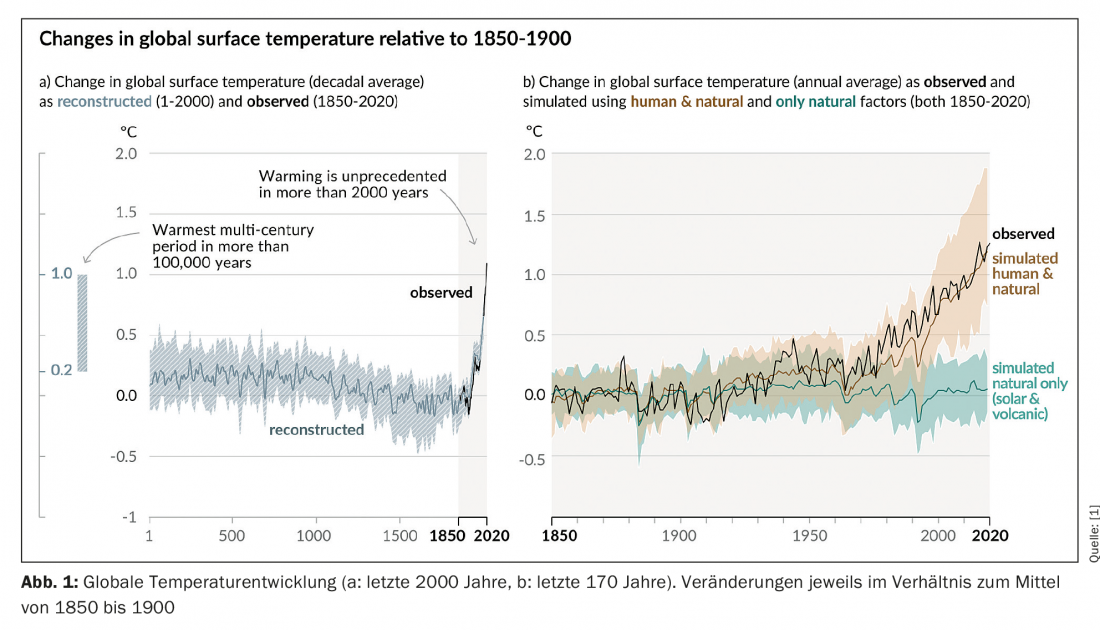
Currently, the global average temperature is about 1 °C above that of the pre-industrial era (Fig. 1) [1]. In Germany, the normal value of the mean annual temperature (average of the years 1971-2000) is about 10 °C and varies quite strongly over the year. In contrast, annual average temperatures in the tropics are quite high (about 25 to 30 °C depending on the region) with little seasonal variation [2]. The IPCC’s current report divides its estimates of global warming into three categories: short-term trend (2021 to 2040), medium-term trend (2041 to 2060), and long-term trend (2081 to 2100). This already illustrates the longer time horizon of climatological processes compared to human scales. These are defined in a way that is completely different in time from human perception and experience, as is evident in the measurement of a span of 20 years as “short-term”. These different temporal scales also play an essential role in the subject of this article.
Given the aforementioned long time horizons in climatological issues, it is not only the unusually steep and constant trend of the increase in global temperature over the last 100 years that is remarkable, but also the acceleration of the establishment of species in new regions due to the – mostly unintentional – anthropogenic carryover associated with globalization. In addition, there are changing dispersal patterns of vectors native here due to current changes in average temperatures.
Prerequisites for tropical vectors
The establishment of tropical vectors as so-called neozoans in the context of such a carryover presupposes that they find suitable habitats and development opportunities as well as suitable hosts (humans or even animals). Even though average temperatures in Europe are rising due to climate change, they are still far from reaching those in the tropics. Thus, introduced tropical vectors must also have a tolerance to less favorable climatic conditions in order to successfully establish themselves here (so-called preadaptation). This excludes some vectors from the outset as candidates for establishment in Europe. The issue of vector-borne tropical diseases in Europe is embedded in a global development on this topic [3–5] and any closer examination must also take into account changes in other parts of the world. The growing importance of vector-borne diseases for public health even beyond the “classic” regions such as sub-Saharan Africa represents a challenge in the future that cannot be ignored [6].
Internal examples of relevant diseases associated with this would be tropical arboviruses such as dengue or chikungunya fever. The most relevant dermatological vector-borne tropical disease in this context is cutaneous leishmaniasis.
A similar case would be the return of vector-borne infections that have since been eliminated in Europe. One example is malaria, which was endemic in northwestern Germany until the late 1940s.
Several example scenarios are used to highlight the propagation potential for vectors. It is also always important to consider the impact of the change in vectors for the diseases associated with them. An increased incidence of vectors is certainly unpleasant and troublesome, but without the necessary pathogens, this is neither a relevant individual medical nor a public health problem.
Scenario: Mosquitoes (Malaria)
This scenario is already unusual in the context of this article, as it is not related to the introduction of tropical vectors and the diseases they transmit. Malaria is a disease that continues to cause particularly high morbidity and mortality [7]. However, Europe is considered malaria-free, but an introduction is always possible via travelers returning from endemic regions. In recent years, approximately 1000 travel-associated malaria cases have been reported annually to the RKI.
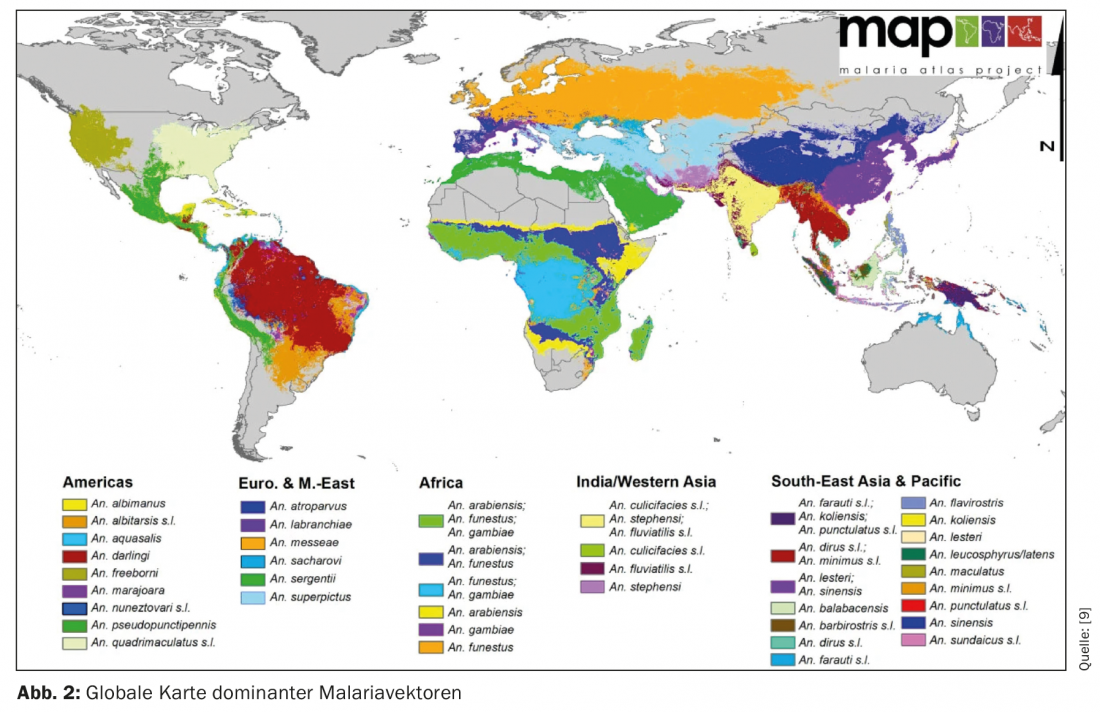
In the context of this article, the question for malaria must now be whether a return of this infection, which has been eliminated in Eu-ro-pa for decades, is conceivable. This does not require the introduction of a tropical vector, but rather the mosquitoes native to our region that used to transmit the disease (mainly Anopheles atroparvus, but also others such as To. messeae) are still native here and are also vector-competent [8]. This leads to the phenomenon of “anophelialism without malaria”. Figure 2 illustrates this with the global distribution of dominant malaria vectors.
In this case, therefore, the pathogens (Plasmodium vivax or Pl. oval) be reintroduced and be able to establish themselves. However, temperature is only one factor here. Especially for the development of plasmodia in mosquitoes, the mean temperature is the crucial factor. Overall, the following additional aspects not associated with climate change should be noted:
Host preference and living conditions: A. atropar-vus as an important vector in Europe prefers animals as hosts (zoophilic), but also bites humans in their absence. In the past, people in rural areas lived much closer together with livestock (sometimes in the same or close buildings), so much more human contact with mosquitoes, which are actually zoophilous, was possible.
Environmental conditions: Environmental change with numerous wetland drainages and management of water bodies has reduced the number of suitable breeding areas.
Public health measures: Effective treatment of infected individuals and targeted control and prevention measures reduce the number of suitable hosts for stable transmission cycles.
In principle, it is possible that plasmodia introduced by travelers – especially in a generally warmer climate – can be picked up by domestic mosquitoes and transmitted in turn. This could affect, above all, southern European countries, which, in view of rising average temperatures, offer better development conditions for at least seasonal cycles. However, provided that the socioeconomic factors (environment, living conditions, and public health in the above list) that have contributed to elimination in Europe remain stable, a permanent return of malaria to Europe is unlikely. Nevertheless, the possibility of at least seasonal cycles in parts of Europe as a result of climate change in subsequent decades underscores the importance of appropriate public health measures. A thorough travel history and, if necessary, a medical examination are required. Diagnostics and therapy, both for individual cases and as a contribution to public health as a whole, are the best measures to prevent diseases or to treat them in a timely manner.
Scenario: Mosquitoes (Arboviros)
Arbovirosis refers to viral diseases transmitted by arthropods. Thus, it is a collective name based on the mode of transmission and not a systematic name with respect to relatedness of these viruses.
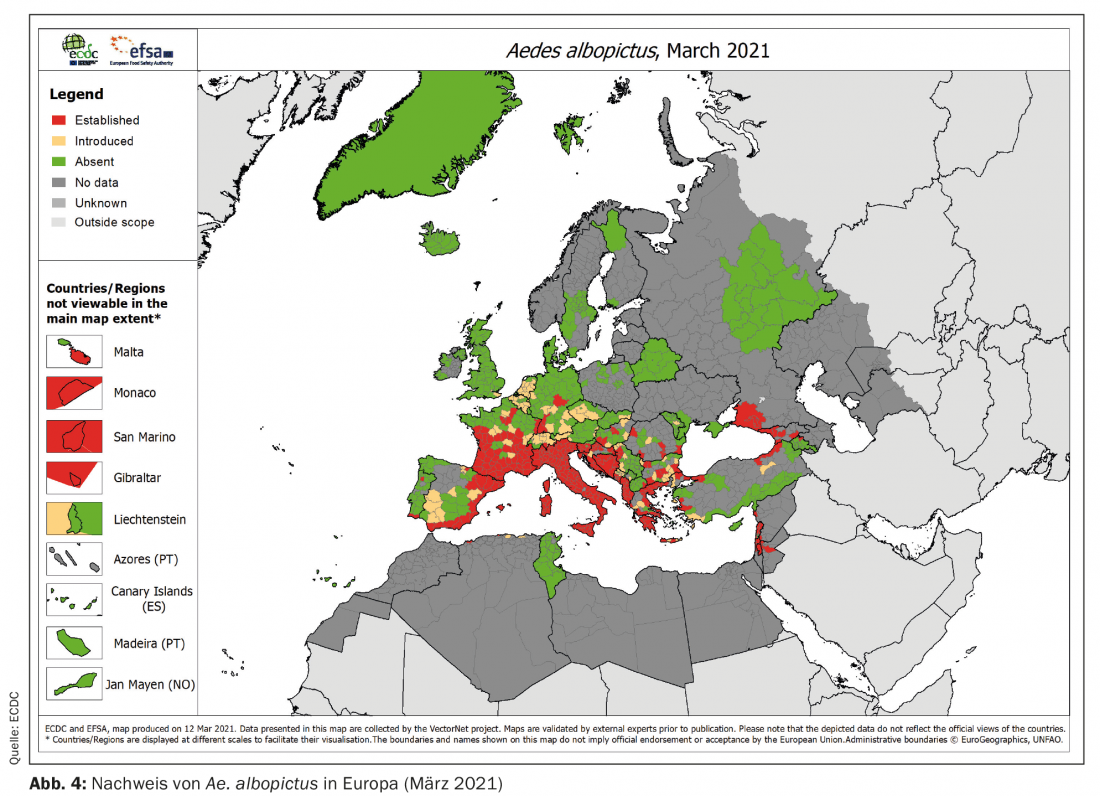
Among tropical arboviroses, dengue and chikungunya fever (DEN and CHIK, respectively) are particularly important in the context of importation and climate change. Both are transmitted in the tropics primarily by the yellow fever mosquito (Aedes aegypti) , but other mosquitoes such as the Asian tiger mosquito (Ae. albopictus) are also suitable vectors. While Ae. aegypti is bound to a permanently tropical-warm climate, is Ae. albopictus more tolerant of cooler climates. With international trade (esp. scrap tire trade), it has been displaced from its original range (Southeast Asia) several times worldwide. In Europe, this species was first recorded in Albania in 1979 (with probably even earlier introductions). In the EU, population trends are monitored through vector surveillance and published by ECDC. From Figures 3 and 4 , it can be seen that Ae. albopictus has spread from almost exclusively Italy to almost the entire Mediterranean region within only 13 years and can now also be found much further north.
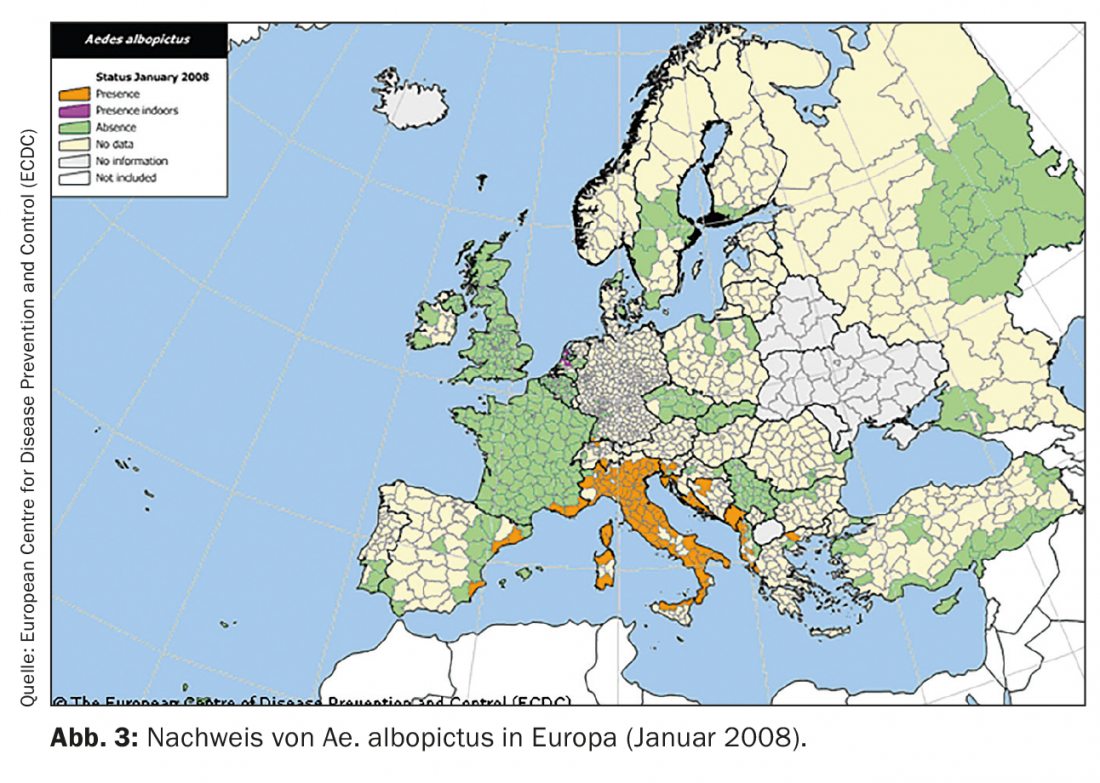
Although both DEN and CHIK are not endemic to Europe, it is repeatedly introduced here by travelers returning to the country. This repeatedly leads to the occurrence of autochthonous cases of DEN and CHIK in the Mediterranean region, as the resident Ae. albopictus are competent vectors and transmit the virus to new hosts. In this respect Ae. albopictus to be considered the first and very successful tropical vector, which has already become firmly established in Europe. However, it is particularly important to note here that this was already happening when anthropogenic climate change was not yet a widely discussed phenomenon.
The competence of Ae. albopictus as a vector of various arboviroses is the reason why ECDC conducts the regular surveillance on their occurrence mentioned above. The species is also a good example of how the presence of a suitable vector alone is not a problem, but is still an essential prerequisite for the establishment of DEN or CHIK, provided they are imported frequently enough to establish stable endemic cycles in the future. For European conditions, it should be noted in particular that DEN is characterized by Ae. albopictus can be transmitted transovarially (i.e., vertically) and thus can survive unfavorable conditions for the vectors (e.g., colder seasons that do not allow mosquito activity).
The appearance of another neozoon – the Japanese bush mosquito (Aedes japonicus) as a suitable vector of West Nile fever – should also be mentioned in this context. It underlines the special potential that arboviroses have in terms of greater spread in Europe as well. Nevertheless, the European range of Ae. japonicus compared with Ae. albopictus still quite small. However, this may also change significantly over the course of the next few years and decades. It should be noted here that it is unrealistic to aim for the elimination of such neozoans, as the experience of other large-scale – and mostly unsuccessful – elimination campaigns regarding vectors in the past has shown. Even in those cases where this was successful (e.g., the eradication of accidentally introduced Anopheles gambiae in Brazil in the 1930s and early 1940s), it involved a large investment of time and resources that is fundable and feasible only if there is an identifiable acute public health problem. However, other mosquito species native to our region are also capable of transmitting West Nile fever, so that its spread is certainly favored by climate change, but this is not a prerequisite.
Scenario: Sandflies (Leishmaniasis)
With regard to leishmaniasis, the situation is similar to that of DEN or CHIK in that the suitable vectors are already indigenous to Europe and do not have to be introduced in the course of climate change. Various sandflies are possible carriers of the disease. As an example, Phlebotomus perniciosus can be mentioned as a vector for Leishmania infantum, the causative agent of cutaneous and visceral leishmaniasis in the Mediterranean region. This is already responsible for regular infections in dogs imported from the Mediterranean region, including to Germany. There is also a social factor to consider here, as dogs cannot be cured and always die from the disease. In particular, initiatives that import stray street dogs from the Mediterranean region for ethical and animal welfare reasons may thus also introduce an infectiological problem. P. papatasi is a competent vector for L. tropica, a causative agent of cutaneous leishmaniasis in North Africa [10] and Asia and is also found in the Mediterranean region.
Sandflies have quite specific requirements especially for temperature, but also humidity, and are thus good examples of possible beneficiaries of a general warming in Europe. Modeling suggests that by the end of the 21st century, both sandflies and leishmaniasis can be expected to occur in central Europe. Figure 5 shows this in terms of the predicted spread of relevant Phlebotomus speciesin Europe by 2070. Again, one recognizes the relatively long period of such changes in population dynamics by human standards.
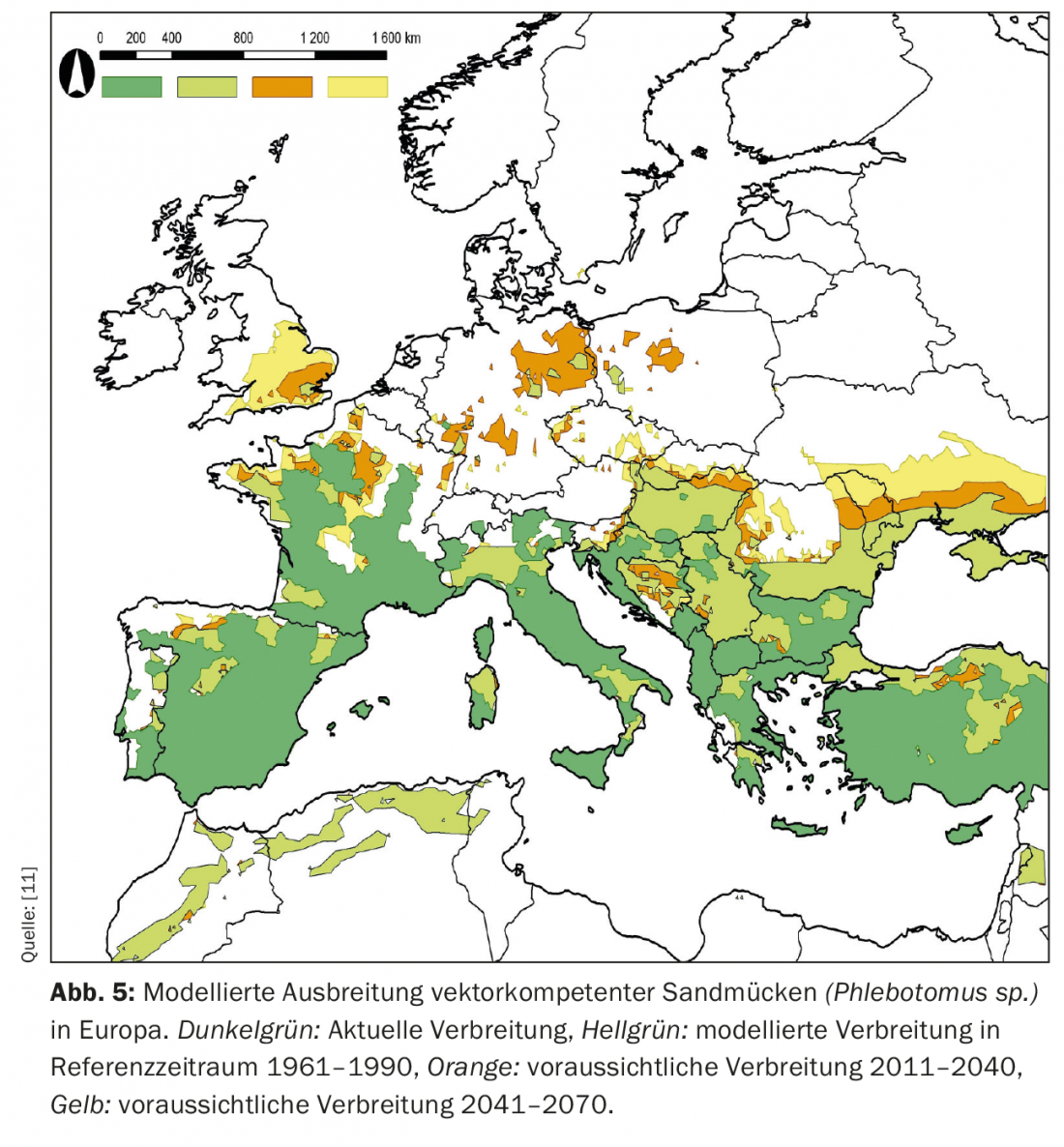
In this sense, it is also in this case not a spread of tropical vectors to Europe, but an expansion of the range of species already established here – but which are capable of transmitting a tropical disease (leishmaniasis) to both dogs and humans.
Scenario: Ticks (TBE, Lyme disease, CCHF)
Ticks, as bloodsucking arthropods, are present almost worldwide. The occurrence of TBE and Lyme disease is nothing unusual in Germany as well as in many other European countries and has been known for a long time. Thus, these vectors may also not fit the topic of this article, but on closer examination, the same mechanisms as e.g. sandflies can be recognized here: Here, too, global (and thus also European) warming is about the changing dispersal areas of native species. Modeling up to the end of this century suggests that the most important vector of TBE and Lyme disease in Europe – the common wood tick (Ixodes ricinus) – may spread further northward in the future. (Fig. 6) This is also likely to be accompanied by declining occurrence in more southern Europe as it becomes warmer and especially drier there.
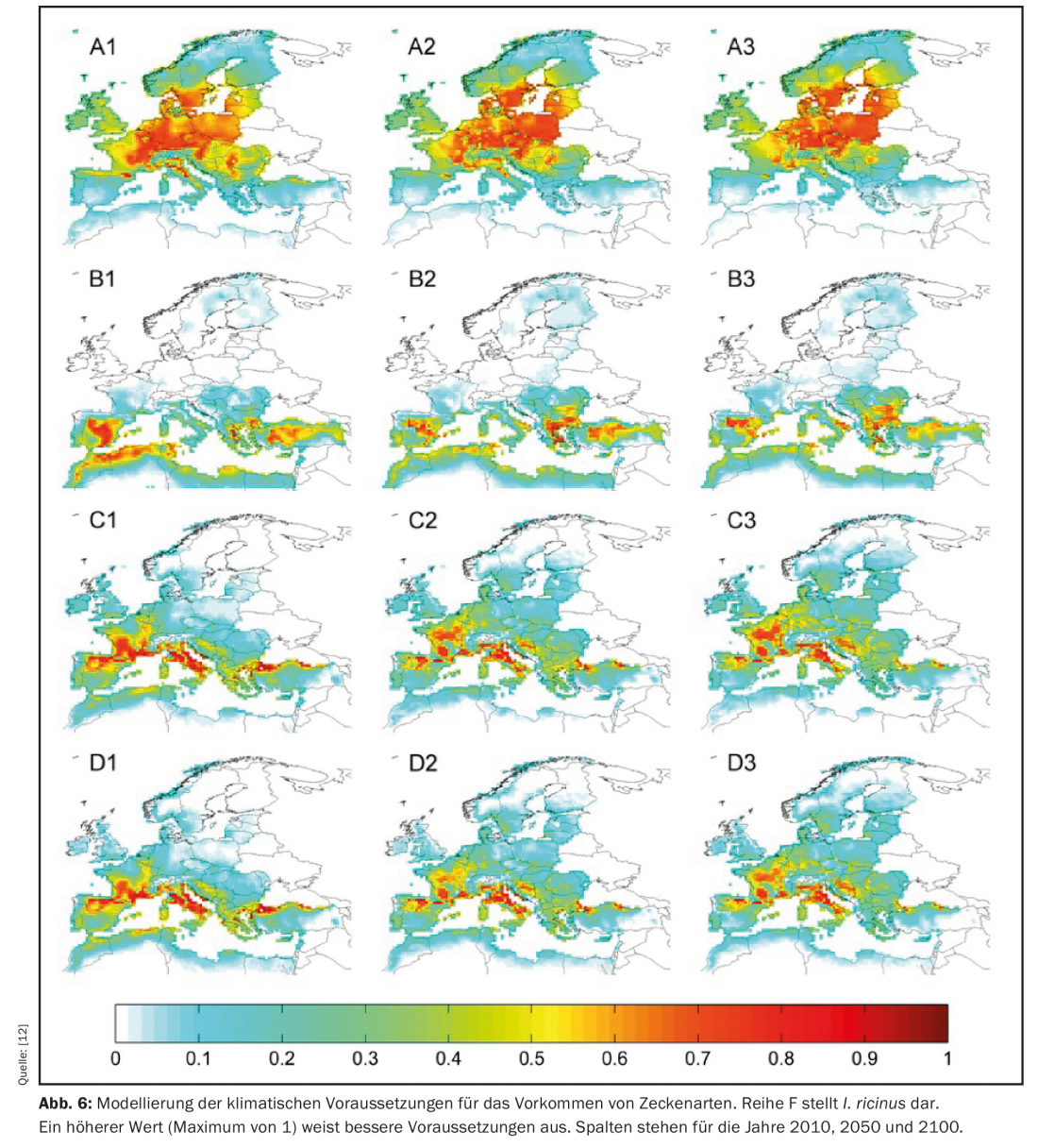
A change in the occurrence of I. ricinus is already evident, in that the species has also been encountered at higher elevations in recent years. This is supported by the increased number of ticks found at higher altitudes as well as by genetic studies, which show no differences between TBE variants from lower or higher altitudes [13]. This phenomenon is interpreted as a result of increasing average temperatures, but also changes in land use and altered residency patterns of wildlife hosts (e.g., red deer or roe deer).
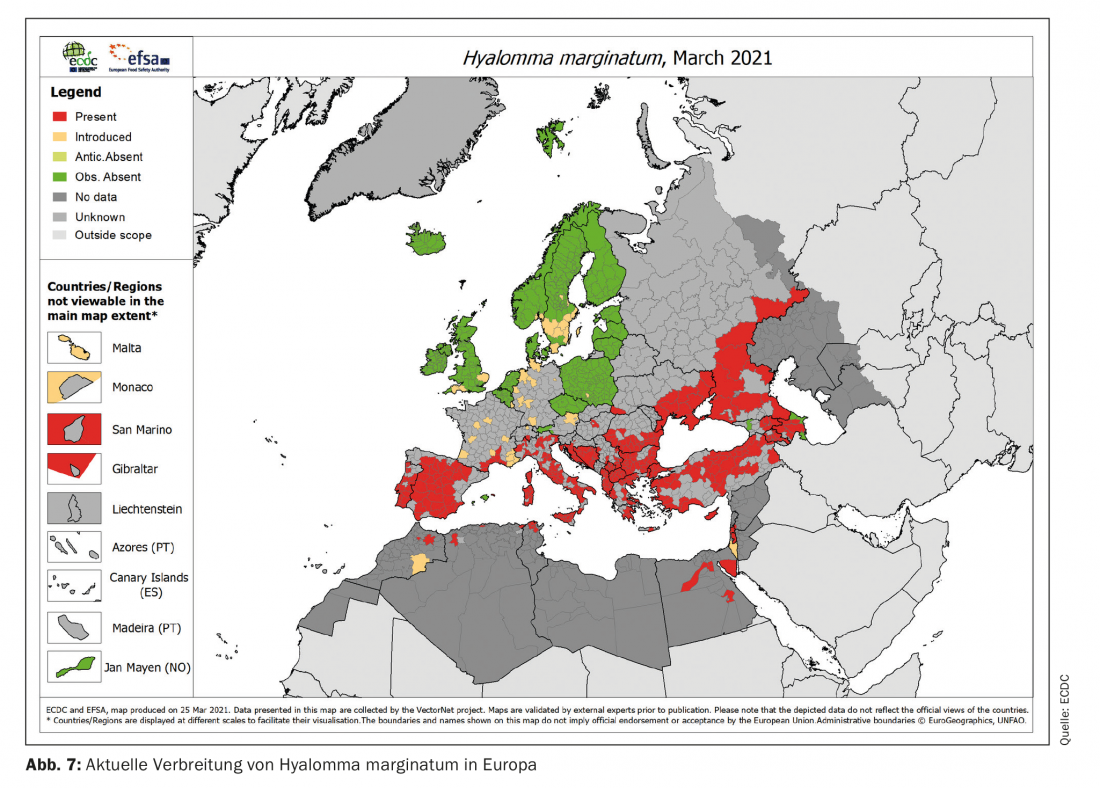
Hyalomma marginatum, the main vector for Crimean-Congo hemorrhagic fever (CCHF) will also be affected by climate change in Europe. The current range (Fig. 7) is expected to shift slightly northward, although it is also likely to shrink in southern regions (e.g., Spain). Overall, a significant expansion of the range in Europe due to climatic changes is not to be expected here. However, the current records also in Germany show the dispersal potential of the species over long distances. Here, different bird species (especially sparrows) or wildlife migrations play a role and underline the non-climatic factors already mentioned in other contexts in the dispersal or spread of vectors. The long residence time of H. marginatum on a host of up to 30 days favors such a spread, so that even the ticks, which are not very mobile due to their lack of flight ability, can be spread relatively quickly over long distances – passively – in this way.
Summary
The preceding examples illustrate that anthropogenic climate change is an important, but not necessarily the decisive, factor in assessing the current and future occurrence of arthropods as vectors of infectious diseases in Europe. Higher temperatures are generally favorable for arthropods as cold-blooded animals, but medical relevance arises only in the context of transmission of vector-associated diseases and aspects of host (human or even animal) behavior. In the absence of pathogens, the increased incidence of hematophagous arthropods may be extremely troublesome, but is not an individual medical or even a public health problem.
Europe’s currently foreseeable challenges with regard to vectors concern not so much the risk of introducing further species into Europe, but the behavior and spread of species already established here. The only currently large-scale relevant species that can be described as a neozoon was introduced to Europe more than 40 years ago. All other relevant vectors are native to Europe. Rising temperatures are also having a measurable impact on the distribution patterns of these species, and this impact will increase in the future.
Human influence on other important factors such as environmental changes, living conditions, and public health interventions plays a critical role in whether or not increased vector emergence also leads to an increase in associated disease.
Take-Home Messages
- Vectors and their occurrence alone are an essential element in risk assessment, but not the only one.
- Climate change leads to changes in the occurrence of species that are already established here, which is why one should regularly check the relevant technical information (e.g. at the RKI or the ECDC).
- Climate change is not the only factor determining an increase in the risk of tropical vectors and the diseases they transmit. Other important factors are: Entomological aspects (host preferences, vector competence); Ecological aspects (presence of suitable habitats); Human intervention (public health and individual medical measures change the availability of suitable hosts and pathogen reservoirs).
- Social factors (settlement and behavioral patterns of the human population alter the likelihood of coming into contact with vectors and pathogens).
- The expected major changes in the occurrence of vectors and (so far) tropical diseases take place over very long periods of time – measured by individual human experience. Significant changes with respect to the risk of such diseases are likely to occur beginning in the mid-21st century.
- The occurrence of competent vectors (esp. Ae. albopictus) for various arboviroses in large parts of Europe plays an important role in travel medicine: travelers returning home can bring pathogens with them and lead to autochthonous cases when stung by such a vector here. Appropriate education on how to behave before, during and after a trip to regions at risk for such diseases is advisable.
Literature:
- Delmotte V, Zhai P, Piran A, et al: Summary for Policymakers. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change 2021; 42.
- Sobel AH.: Tropical Weather. Nature Education Knowledge 2012; 3: 2.
- Caminade C, McIntyre KM, Jones AE: Impact of recent and future climate change on vector-borne diseases. Annals of the New York Academy of Sciences 2019; 1436: 157-173.
- Watts N, Amann M, Arnell N, et al: The 2020 report of The Lancet Countdown on health and climate change: responding to converging crises. The Lancet 2021; 397: 129-170.
- Fouque F, Reeder JC: Impact of past and on-going changes on climate and weather on vector-borne diseases transmission: a look at the evidence. Infectious Diseases of Poverty 2019; 8: 51.
- Rocklöv J, Dubrow R: Climate change: an enduring challenge for vector-borne disease prevention and control. Nature Immunology 2020; 21: 479-483.
- WHO: World malaria report 2020: 20 years of global progress and challenges. Geneva 2020.
- Hertig E: Distribution of Anopheles vectors and potential malaria transmission stability in Europe and the Mediterranean area under future climate change. Parasites & Vectors 2019: 12: 18.
- Sinka ME, Bangs MJ, Manguin S, et al: A global map of dominant malaria vectors. Parasites & Vectors 2012; 5: 69.
- Aoun K, Bouratbine A: Cutaneous leishmaniasis in North Africa: a review. Parasite 2014; 21: 14.
- Trájer A, Bede-Fazekas Á, Hufnagel L, et al: The effect of climate change on the potential distribution of the European Phlebotomus species. Applied Ecology and Environmental Research 2013; 11: 189-208.
- Williams HW, Cross DE, Crump HL, et al: Climate suitability for European ticks: assessing species distribution models against null models and projection under AR5 climate. Parasites & Vectors 2015; 8: 440.
- Lemhöfer G, Chitimia-Dobler L, Dobler G, Bestehorn-Willmann M: Comparison of whole genomes of tick-borne encephalitis virus from mountainous alpine regions and regions with a lower altitude. Virus Genes 2021; 57: 217-221.
DERMATOLOGIE PRAXIS 2021; 31(5): 10-16