Psoriasis is nowadays no longer understood as an exclusive skin disease, but rather as a systemic disease. Biologics specifically intervene in the disease process and have revolutionized treatment options. When selecting the individually suitable active substance, there are a number of things to consider.
In Switzerland, psoriasis affects about 2% of the population [1]. Chronic systemic skin disease manifests as plaque psoriasis in 90% of cases and leads to significant limitations in quality of life [1,2]. Affected individuals are at increased risk of developing comorbidities, such as cardiovascular disease, depression, metabolic syndrome, joint disease, and inflammatory bowel disease (Box) [2]. Nowadays, psoriasis is understood as an inflammatory systemic disease in which proinflammatory cytokines play an important role, explains Prof. Thomas M. Kündig, MD, Director of the Department of Dermatology at the University Hospital Zurich [3].

Complex systemic disease
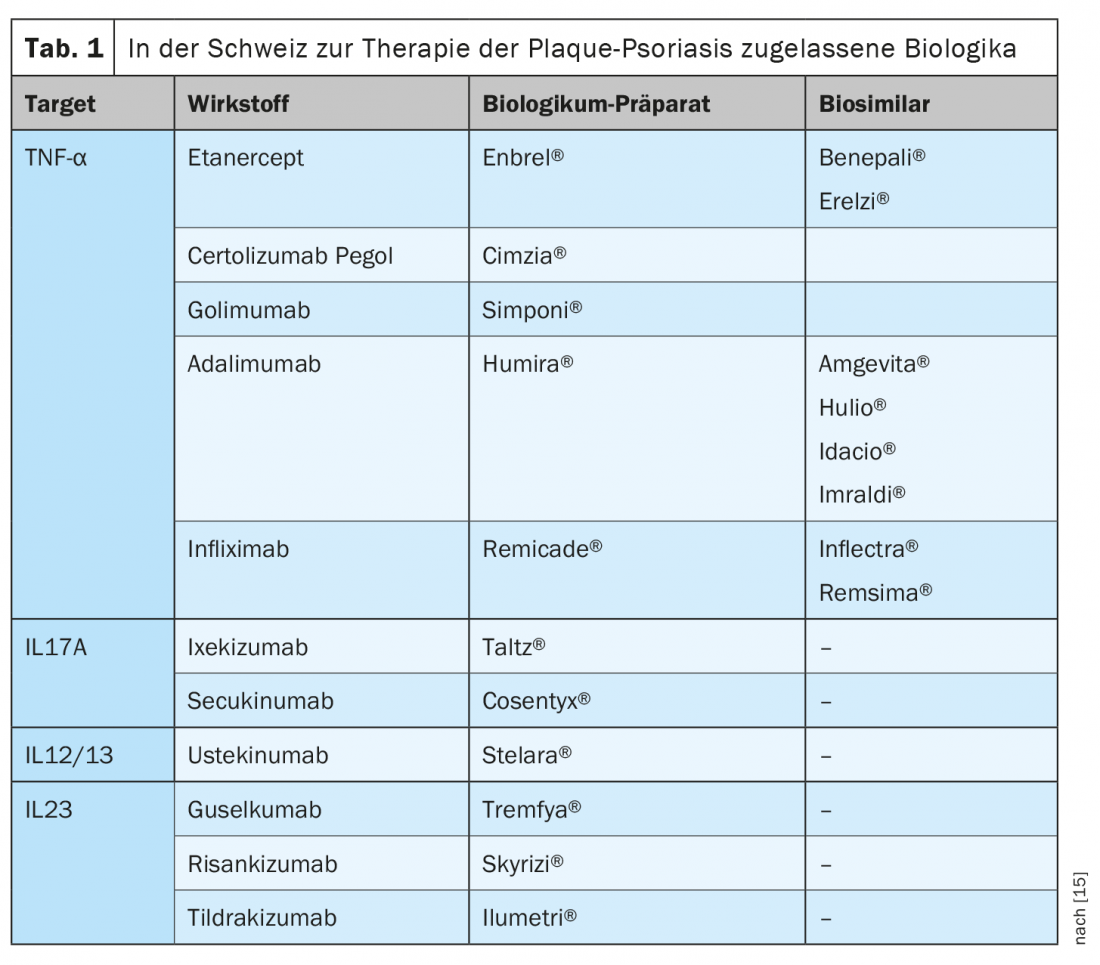
That there are correlations with cardiovascular diseases, among other things, has been shown by various research findings in recent years. Prof. Kündig refers to a widely cited population-based British study published about fifteen years ago, in which Gelfand et al. Data from patients with psoriasis (n=130 976) compared with healthy controls (n=556 995), [4]. The age range was 20 to 90 years, and in the group of psoriasis patients there were 127 139 with a mild course and 3837 with a severe course. Psoriasis was found to be an independent risk factor for myocardial infarction, with the highest risk affecting younger patients with severe psoriasis. The relative risk (RR) for myocardial infarction was 1.29 (95% CI; 1.14-1.46) for a 30-year-old patient with mild psoriasis and 3.10 (95% CI; 1.98-4.86) for severe psoriasis, so severity plays an important role. Over time, researchers came to an increasingly sophisticated understanding of the molecular basis of these relationships. Today, it is assumed that psoriasis can be characterized both by components of an immune disease and those of an autoinflammatory disease, according to Prof. Kündig. Psoriasis is therefore an inflammatory systemic disease, so that comorbidities must also be taken into account in treatment. The therapeutic landscape has changed, especially for moderate and severe plaque psoriasis, and the demands on the treatment outcome have increased. Nowadays, biologics make freedom from manifestations and restoration of quality of life realistic therapeutic goals (Overview 1, Tab. 1).

Individual therapy strategy
With the biologic therapies available today, patients with moderate to severe plaque psoriasis can achieve rapid and lasting symptom relief and a sustained improvement in quality of life. In view of the variety of approved substances (Tab. 1), a criterion-guided decision as to which therapy is best suited for which patient is becoming increasingly important. In addition to the original biologics, so-called biosimilars exist for some of the substances that have already been approved for at least twenty years (period of patent protection). For approval of a biosimilar, manufacturers must demonstrate that the product is sufficiently similar to the reference product in terms of structure, pharmaceutical quality, biological activity, efficacy, safety and immunogenicity to exclude relevant clinical differences with sufficient certainty [5].
Increasingly, the goal is to provide personalized treatment tailored to patient characteristics. This requires understanding in which patients the available drugs should be used and how. An analysis of large patient registries such as PsoBest, PsoNet or the Swiss Dermatology Network for Targeted Therapies (SDNTT) provides a good basis for individualized therapy (box). In the Swiss Psoriasis Registry (SDNTT), an association between comorbidities, psoriasis disease severity, and systemic therapies was found [6]. A Belgian study published in JEADV 2020 used an expert consensus process to formulate practice recommendations for biologic therapy in plaque psoriasis [7]. The following is an excerpt from it:
Implications of metabolic comorbidities: In psoriasis patients with metabolic syndrome, careful monitoring and follow-up is recommended, including control of weight, blood pressure, triglycerides, HDL cholesterol, and glucose levels. Studies show that weight-adjusted dosing is required in obese patients with respect to the biologics ustekinumab, infliximab, adalimumab, and etanercept. With regard to the newer substances such as risankizumab, guselkumab, ixekizumab, and brodalumab, the data base is not yet as large, but there are indications that the weight of the patients has less influence on the effectiveness of the therapy with these biologics [8–10,22]. Furthermore, the nutrition/weight control factor should be addressed in obese psoriasis patients [11].
Biologics-experienced vs. biologics-naïve: There is evidence that in patients who have had at least one biologic therapy, switching to a different drug class results in better drug survival than switching within the same class [12]. However, this is put into perspective when the factors of efficacy, side effects, and comorbidities are included. In this regard, there is evidence that switching within the same class of biologics may also be a good option [13]. According to current data, it is less important for the newer biologics whether a previous therapy attempt with another biologic was unsuccessful [14].
Mental health factor: patients with psoriasis are more likely to experience depression, anxiety disorders, suicidality, sleep disturbances, and reduced quality of life, and it is known that these symptoms can be reduced with effective treatment [23]. The expert panel therefore advises to initiate adequate effective treatment as soon as possible. In one large study, biologic therapy was associated with a reduction in antidepressant use [16,17].
Congress: USZ Update Therapies
Literature:
- Navarini AA, et al: Estimation of cost-of-illness in patients with psoriasis in Switzerland. Swiss Med Wkly, 2010. 140(5-6): 85-91.
- Boehncke WH, et al: Psoriasis. Lancet, 2015. 386(9997): 983-994.
- Kündig TM: Update Therapies: Skin cancer and inflammatory skin diseases. Prof. Dr. Thomas M. Kündig, 10.12.2020
- Gelfand JM, et al: Risk of Myocardial Infarction in Patients With Psoriasis. JAMA. 2006; 296(14): 1735-1741.
- Swissmedic: Wegleitung Zulassung Biosimilar HMV4. www.swissmedic.ch (last accessed 09.03.21)
- Cazzaniga S, et al: Linkage between patients’ characteristics and prescribed systemic treatments for psoriasis: a semantic connectivity map analysis of the Swiss Dermatology Network for Targeted Therapies registry. JEADV 2019; 33(12): 2313-2318.
- Lambert JLW, et al: Practical recommendations for systemic treatment in psoriasis according to age, pregnancy, metabolic syndrome, mental health, psoriasis subtype and treatment history (BETA-PSO: Belgian Evidence-based Treatment Advice in Psoriasis; part 1). JEADV 2020; 34(8): 1654-1665.
- Timmermann S, Hall A: Population pharmacokinetics of brodalumab in patients with moderate to severe plaque psoriasis. Basic Clin Pharmacol Toxicol 2019; 125: 16- 25.
- Gordon KB, et al: Efficacy of guselkumab in subpopulations of patients with moderate-to-severe plaque psoriasis: a pooled analysis of the phase III VOYAGE 1 and VOYAGE 2 studies. Br J Dermatol 2018; 178: 132-139.
- Khatri A, et al: Pharmacokinetics of risankizumab in Asian healthy subjects and patients with moderate to severe plaque psoriasis, generalized pustular psoriasis, and erythrodermic psoriasis. J Clin Pharmacol 2019; 59: 1656-1668.
- Al-Mutairi N, Nour T: The effect of weight reduction on treatment outcomes in obese patients with psoriasis on biologic therapy: a randomized controlled prospective trial. Expert Opin Biol Ther 2014; 14: 749-756.
- Cozzani E, et al: Serial biologic therapies in psoriasis patients: a 12-year, single-center, retrospective observational study. J Am Acad Dermatol 2019; 82: 37-44.
- Kimmel G, et al: Brodalumab in the treatment of moderate to severe psoriasis in patients when previous anti-interleukin 17A therapies have failed. J Am Acad Dermatol 2019; 81: 857-859.
- Wang TS, Tsai TF: Biologics switch in psoriasis. Immunotherapy 2019; 11: 531-541.
- Swissmedic: www.swissmedicinfo.ch (last accessed 09.03.21).
- Wu CY, et al: Depression and insomnia in patients with psoriasis and psoriatic arthritis taking tumor necrosis factor antagonists. Medicine (Baltimore) 2016; 95: e3816.
- Strober B, et al: Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol 2018; 78: 70-80.
- PsoBest, www.psobest.de, (last accessed 09/03/21).
- PsoNet, www.psonet.de, (last accessed 09.03.21).
- SDNTT, https://my.derma.ch/spec/SDNTT.html, (last accessed 09.03.21).
- Richter L: Psoriasis – a widespread disease. DFP Literature Studies 2020, https://oeadf.at/images/e-learning/01_DFP_Psoriasis_20200915_OEADF.pdf
- Reich K, et al: The effect of bodyweight on the efficacy and safety of ixekizumab: results from an integrated database of three randomised, controlled phase 3 studies of patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol 2017; 31: 1196-1207.
- Gordon KB, et al: Anxiety and depression in patients with moderate-to-severe psoriasis and comparison of change from baseline after treatment with guselkumab vs. adalimumab: results from the phase 3 VOYAGE 2 study. JEADV 2018; 32: 1940-1949.
DERMATOLOGIE PRAXIS 2021; 31(2): 44-45 (published 4/14-21, ahead of print).