In patients with psoriatic arthritis who do not respond adequately to conventional DMARDs or a biologic, the EULAR recommendations advise the use of a JAK inhibitor. Recently, upadacitinib, another member of this drug group, received an indication extension for psoriatic arthritis. This approval is based on the SELECT-PsA 1 and 2 studies.
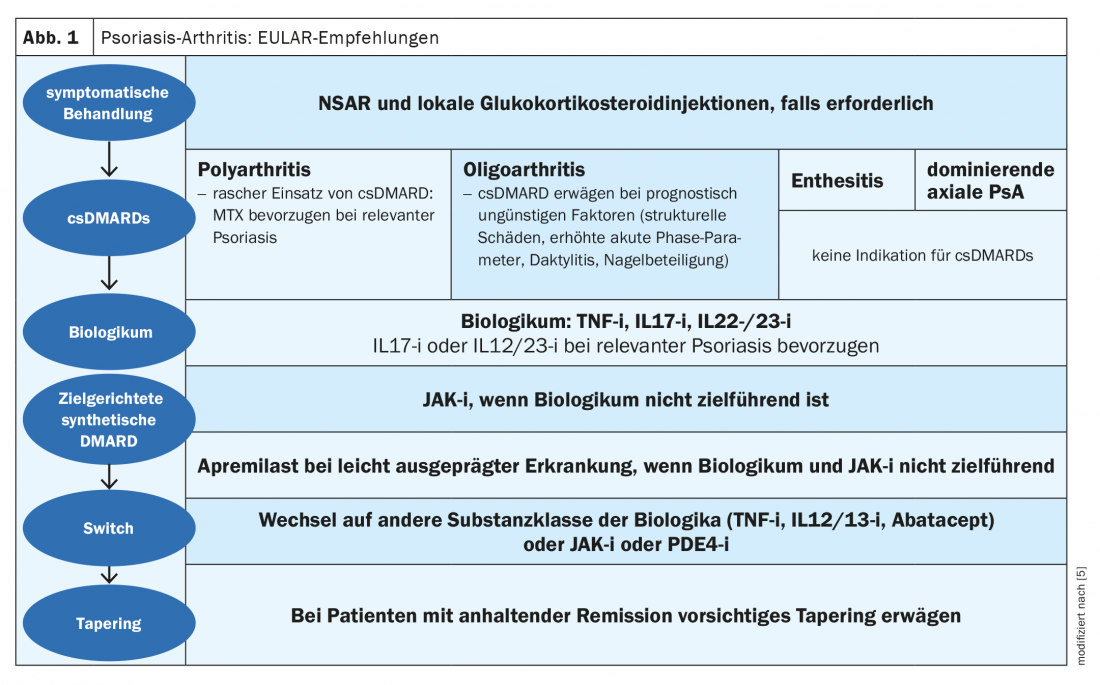
Up to 30% of patients with plaque psoriasis develop psoriatic arthritis (PsoA) during the course of the disease [1]. The CASPAR classification (“Classification Criteria for Psoriatic Arthritis”) is used for diagnosis. This diagnostic tool detects sacroilitis, enthesitis, dactylitis and nail infestation, among others. Whether the JAK inhibitor upadacitinib (Rinvoq®), approved in Switzerland since January 2020 for the treatment of rheumatoid arthritis, is also effective in other diseases with joint involvement, such as psoriatic arthritis and ankylosing spondylitis, has been the subject of several clinical trials [2]. Upadacitinib is an oral Janus kinase (JAK1)-selective inhibitor. In the EULAR recommendations, JAK inhibitors are proposed as a treatment option for psoriatic arthritis in case of failure of a first biologic (Fig. 1) [5].
SELECT-PsA 1 and 2
After the results of the randomized, double-blind, placebo-controlled phase III SELECT-PsA 2 trial were presented on the online EULAR last year, publication of the full-length data from Mease et al. Published [3,4]. While the SELECT-PsA 1 trial involved a head-to-head comparison with adalimumab, the smaller SELECT-PsA 2 trial evaluated upadacitinib in adult patients with active PsoA (SJC ≥3 and TJC ≥3) according to CASPAR criteria and failure or intolerance of ≥1 bDMARD. In the study, 642 patients (54% women, mean age 53 years, duration since PsoA diagnosis 10 years) were randomized 2:2:1:1 for 24 weeks to 1× daily upadacitinib 15 mg or 30 mg or placebo (stratified by csDMARD use, number of prior bDMARDs, and degree of psoriasis), then placebo patients switched to upadacitinib 15 mg or 30 mg. 61% of participants had inadequate response to one bDMARD, 18% to two, and 13% to ≥3, mean SJC66 (“Swollen Joint Count” with 66 joints) and TJC68 (“Tender Joint Count” with 68 joints) at baseline were 12 and 25, respectively.
Relief of joint and skin symptoms
Primary endpoint was ACR20 response at week 12; secondary endpoints included ACR50/70 response, PASI 75/90/100 response, achievement of minimal disease activity (MDA), and resolution of enthesitis and dactylitis. The best treatment results were consistently achieved with the 30 mg dose, which was also shown to be superior to adalimumab in SELECT-PsA 1 (ACR20, HAQ-DI, and pain) [3,4]. At week 12, significantly more patients receiving upadacitinib 15 mg and 30 mg achieved an ACR20 response (56.9% and 63.8% vs. 24.1%, respectively; p<0.0001 each). A similar result was seen for ACR50/70 response (31.8% and 37.6% vs. 4.7% and 8.5% and 16.5% vs. 0.9%, respectively; p<0.05 each). Significantly better performance was also seen by both upadacitinib arms in all other secondary endpoints at week 12, such as ΔHAQ-DI (-0.30 and -0.41 vs. -0.10), ΔSF-36 PCS (5.2 and 7.1 vs. 1.6), and ΔFACIT-F (5.0 and 6.1 vs. 1.3) (each p<0,0001). Also, in skin response at week 24 (PASI 75/90/100: 53.8% and 62.6% vs. 19.1%; 36.2% and 46.6% vs. 6.9%; 22.3% and 33.6% vs. 4.6%, respectively) and symptom reduction of enthesitis (LEI=0: 39% and 48% vs. 20%, respectively) and dactylitis (LDI=0: 64% and 76% vs. 36%, respectively) at week 12, upadacitinib 15 mg and 30 mg was significantly superior in a placebo comparison. The safety profile of upadacitinib was in line with expectations, and all treatment-emergent adverse events were slightly more frequent with the 30 mg dose than with upadacitinib 15 mg or placebo. Severe infections occurred in 0.5% of study participants on placebo and upadacitinib 15 mg and 2.8% on upadacitinib 30 mg, respectively, and herpes zoster developed in 0.9%, 1.4%, and 3.7%, respectively.

Based on the results of the SELECT-PsA 1 and 2 studies, upadacitinib has recently received an indication extension for psoriatic arthritis in Switzerland (box) . Overall, upadacitinib is the first and currently the only JAK inhibitor that can be used in rheumatoid arthritis, as well as in ankylosing spondylitis and psoriatic arthritis [2,7]. To minimize the risk of side effects, patients’ vaccination protection should be updated before starting therapy according to current vaccination recommendations [6]. Use of live attenuated vaccines is not recommended during or immediately prior to initiation of treatment with upadacitinib [6].
Literature:
- Chicken CK, et al: JDDG 2019; 17(1): 43-66.
- Swissmedic: www.swissmedic.ch (last accessed 29.03.2021)
- Rheumamanagement-online.de: Psoriasis Arthritis: www.rheumamanagement-online.de, 01.03.2021, (last retrieved 29.03.2021)
- Mease PJ: Psoriatic arthritis: upadacitinib for psoriatic arthritis refractory to biologics: SELECT-PsA 2 Rheum Dis 2020; doi: 10.1136/annrheumdis-2020-218870
- Lunzer R, Nothnagl T: Eular 2020: Brief overview – Practical relevance …! Rheumatism Plus. 2020 Sep 9: 1-3.
- Physician Information Brochure, Version 2.0, January 2021, https://www.bfarm.de, (last accessed 03/31/21).
- Keim J, www.medizinonline.ch/artikel/neue-therapieoption-bei-ankylosierender-spondylitis-und-psoriasis-arthritis-1, (last accessed 03/31/21).
- van der Heijde D, et al: Efficacy and safety of upadacitinib in patients with active ankylosing spondylitis (SELECT-AXIS 1): a multicentre, randomised, double-blind, placebo-controlled, phase 2/3 trial. Lancet, 2019. 394(10214): 2108-2117.
- McInnes I, et al: Efficacy and safety of upadacitinib versus placebo and adalimumab in patients with active psoriatic arthritis and inadequate response to non-biologic disease modifying anti-rheumatic drugs (SELECT-PsA-1): a double-blind, randomized controlled phase 3 trial [abstract LB0001]. Ann Rheum Dis 2020. 79(Suppl 1); 16: 2-17.
DERMATOLOGIE PRAXIS 2021; 31(2): 46-47