In recent years, it has been recognized that therapeutic success depends crucially on early disease-modifying therapy (DMARD), which can bring disease activity under control. This requires collaborative multidisciplinary care. The arsenal of active ingredients has grown considerably in recent years. Biologics and “small molecules” enable targeted and individualized treatment strategies. According to EULAR, their use can be alternative to or combined with conventional DMARDs.
“In the field of arthritis therapies, there have been huge advances in treatment in recent years,” explains Thomas Langenegger, MD, Chief Medical Officer, Zug Cantonal Hospital [1]. The advancement of pharmacotherapy and the early use of patient-adapted treatment have significantly improved the prognosis in rheumatoid arthritis in recent decades. Modern disease-modifying active substances specifically intervene in the inflammatory processes. According to the European League Against Rheumatism (EULAR), the treatment goal of sustained remission or at least low disease activity can now be achieved in 70-80% of all patients with rheumatoid arthritis. This has positive effects on the quality of life and the preservation of independence for those affected [2].
Interdisciplinary diagnostic clarification
A rethink has taken place in the treatment of immunologically caused rheumatic diseases. “There is increasing evidence that the earlier and more targeted the therapy is and leads to remission, the sooner the drugs can be reduced or, in some cases, even discontinued in the course,” the expert explains. Cytokine-mediated immunopathological pathways, whose activation induces inflammation in tissues and joints, play an important role in pathogenesis. Typical symptoms of rheumatoid arthritis are pain, joint stiffness and restricted movement. Diagnosis is made based on history and the ACR/EULAR classification criteria, as well as other findings from inspection, laboratory, ultrasound, and radiographic examinations [3]. Since joint damage in rheumatoid arthritis can progress rapidly, it is important to make a confirmed diagnosis as early as possible. In addition to remission, the therapeutic goal is to prevent joint destruction or organ damage and to alleviate any comorbidities (box) . The drugs used to treat arthritis today are divided into the following three classes: NSAIDs (non-steroidal anti-inflammatory drugs), glucocorticoids and basic drugs. “There are more and more drugs and therapeutic options for more and broader indications,” Dr. Langenegger said.

Use glucocorticoids for a limited time
Steroids still play an important role in the treatment of rheumatoid arthritis, especially in the induction phase of therapy until the basic medications achieve their full efficacy. But nowadays, steroids are only given for as long as necessary, or as short a time as possible, and at the lowest possible dose, explains Dr. Langenegger. In the long-term perspective, one usually tries to phase out steroids altogether. However, there are certain indications or patient groups where this is not possible. In these cases, it is recommended to keep the therapy as low-dosed as possible and especially to think about the most common relevant side effect of long-term steroid therapy: osteoporosis. In addition to bone density measurements, prophylaxis with calcium and vitamin D may be considered in these patients, as well as therapy with antiresorptive biphosphonates if needed.
NSAIDs are also still used today, with proton pump inhibitors (PPIs) being used in long-term therapy for prophylaxis of undesirable gastrointestinal side effects. If COX2 inhibitors are used, potential cardiovascular side effects should be considered, the speaker said.
EULAR recommendations advise early use of DMARDs
Basic therapy involves the use of anti-inflammatory disease-modifying anti-rheumatic drugs (DMARDs), which have significantly fewer side effects than NSAIDs and corticosteroids. They alter the course of the disease by targeting the inflammatory signaling pathways in the immune system, slowing down and, in the best case, halting the inflammatory process, which subsequently reduces symptoms [4]. Currently available on the market are conventional disease-modifying agents (csDMARDs), biologics and biosimilars (bDMARDs), and partially synthetic agents (tsDMARDs). In 2020, the European League Against Rheumatism (EULAR) published an update of its treatment recommendations for the management of rheumatoid arthritis (RA) [8,10]. It recommends starting therapy with DMARDs as soon as possible after diagnosis. As recent studies have shown, rapid control of disease activity has a positive impact on further prognosis [5–7]. Methotrexate (MTX) is still considered the first choice, supplemented as needed by short-term use of oral corticosteroids, especially at the start of therapy or a change of conventional DMARD due to insufficient response. Leflunomide or sulfasalazine can be used as alternatives to MTX. Patients who respond inadequately to initial DMARD therapy should be stratified according to unfavorable prognostic factors. These factors include autoantibodies, persistent moderate or high disease activity, early erosions, and high numbers of swollen joints. In the absence of adverse prognostic factors, switching csDMARDs should be considered. In high-risk patients, the additional use of a biologic (bDMARD) or tsDMARDs is recommended at an early stage. Combined use with MTX should be preferred over monotherapy. If an adequate response is not achieved with combination therapy, a switch to another bDMARD or another tsDMARD with a different mechanism of action should be made.

More and more biologics and representatives of the “small molecules” available
Rituximab, the first representative of the biologics (bDMARDs) for the treatment of rheumatism, was approved for marketing in 1997. Since then, numerous other active agents have been added (Tab. 1) . In addition to TNF-alpha inhibitors, IL6 inhibitors and antibodies directed against B cells are available. Biologics are derived from or with the help of biological organisms (e.g. cell cultures, bacteria), have a complex structure and a complex manufacturing process. Therefore, the cost of this group of drugs is relatively high. For some years now, biosimilars have also been on the market. These are copycat products with a high degree of similarity in terms of structure, biological activity, purity and safety.
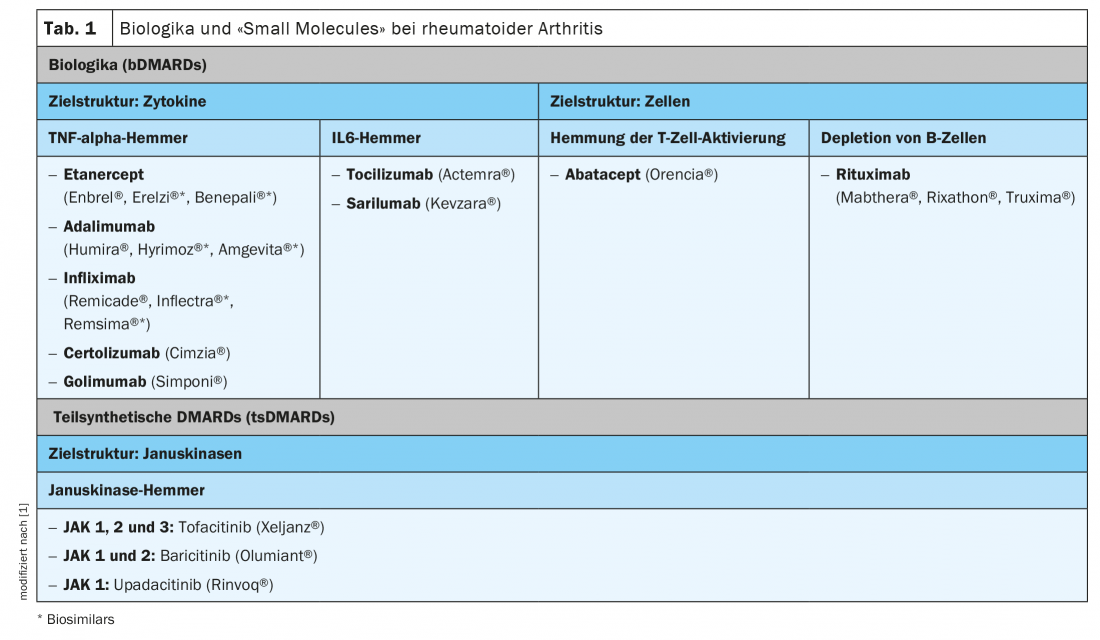
Semi-synthetic disease-modifying agents (tsDMARDs) are small molecules that specifically inhibit the synthesis or expression of certain cytokines intracellularly. In 2013, tofacitinib, the first of the Janus kinase inhibitors, was approved for rheumatoid arthritis, followed later by baricitinib and upadicitinib (Table 1). Filgocitinib and peficinitib are currently being investigated in pivotal clinical trials. Janus kinases (JAKs) are intracellular enzymes that are activated via the production of cytokines at cell surface receptors. Activated JAKs lead to the production of proinflammatory cytokines via STAT and through the nucleus.
Source: FomF General and Internal Medicine
Literature:
- Langenegger T: Arthritis Therapy: Thomas Langenegger, MD, Update. FOMF 2.12.2020.
- Rheumatoid arthritis: living with a chronic disease. Rheumatism League Switzerland 2020, www.rheumaliga.ch/assets/doc/ZH_Dokumente/Broschueren-Merkblaetter/Krankheitsbilder/RA.pdf
- Aletaha D, et al: 2010 Rheumatoid Arthritis Classification Criteria, Arthritis & Rheumatism, Vol. 62, No. 9, September 2010, pp 2569-2581, retrieved 28 Aug 2019.
- Schneider M, et al: Guideline on the management of early rheumatoid arthritis. German Society of Rheumatology 4th edition, 2019.
- Combe B, et al: Annals of the rheumatic diseases 2017; 76: 948-959.
- Emery P: Br J Rheumatol 1995; 34(Suppl 2): 87-90.
- Machold KP, et al: J Rheumatol Suppl 1998; 53: 13-19.
- RheumaGuide: The information service for rheumatologists, www.rheumaguide.de/content/update-zur-behandlung-von-ra-patienten
- FAQ, Coronavirus, www.rheumaliga.ch/blog/2021/coronavirus-haeufig-gestellte-fragen?q=biologika
- Smolen JS, et al: EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update Ann Rheum Dis 2020; 79: 685-699.
HAUSARZT PRAXIS 2021; 16(2): 34-35 (published 2/19-21, ahead of print).