Benign liver lesions are common findings on abdominal imaging. In most cases, they cause no symptoms and are discovered by chance. Therefore, it is difficult to provide valid prevalence data. In studies with large cohorts, liver lesions are found in about 6-10% of cases, and the number increases to about 15% when focal adipose disorders are included.
Benign liver lesions are common findings on abdominal imaging. In most cases, they cause no symptoms and are discovered by chance. Therefore, it is difficult to provide valid prevalence data. In studies with large cohorts, liver lesions are found in about 6-10% of cases, and the number increases to about 15% when focal adipose disorders are included [1]. The diversity of benign liver lesions presents a diagnostic challenge. Etiologic assignment is important because the clinical management of each entity differs markedly [2].
Sonography plays a significant role in the diagnosis of focal liver lesions [3]. First, it is often the method by which the changes are primarily detected, whether as part of “screening” examinations or those performed because of elevated liver values or abdominal pain. On the other hand, sonography is the first method to further characterize a liver lesion due to its good availability and lack of contraindications. To increase the diagnostic value of gray-scale and duplex sonography, the use of contrast-enhanced ultrasound (CEUS) has been standard for several years to differentiate liver lesions [3]. The author is always amazed that on the one hand the diagnostic accuracy of the method is proven by large studies and its use is recommended by guidelines [4,5]. But on the other hand, neither the level of awareness of the method among physicians and students nor the availability of KM ultrasonography so far do justice to this fact.
Because solid focal liver lesions cannot ultimately be clearly assigned etiologically by gray-scale and duplex ultrasonography, the use of contrast agents significantly increases the value of ultrasonography as a method. It should not be forgotten that alternative imaging modalities (CT, MRI) also rely on contrast agents to achieve sufficient diagnostic accuracy. In contrast to CT or MRI contrast media, the only ultrasound contrast medium approved in Germany, Austria and Switzerland, SonoVue® (sulfur hexafluoride), can also be used in advanced renal insufficiency, as it is not eliminated renally but exhaled.
A major advantage of KM ultrasonography is that perfusion can be visualized in real time (real-time examination). In addition to comparing the contrast intensity of a mass with the surrounding parenchyma at different stages of the examination (as in CT and MRI), this also allows an assessment of the vascular architecture. In cases where no clear etiologic assignment is possible, the dual blood supply to the liver (arterial and portal venous) means that a distinction between benign and malignant lesions can at least be made with a high degree of certainty.
The most common sonographically detected benign liver lesions are cysts, fatty disorders, hemangiomas, and focal nodular hyperplasia [1]. In addition to findings primarily noted during an ultrasound examination, it is common for space-occupying lesions detected on CT to require sonographic correlation. The domain of gray-scale sonography is primarily the detection of cystic changes. Therefore, these will be discussed separately from solid benign hepatic space lesions below.
Cystic liver lesions
The term “cyst” is often used, but strictly speaking it is not an entity, but a collective term. It is therefore recommended to use the term dysontogenetic hepatic cyst for a cyst in the strict sense. In contrast, the term cystic liver lesion may subsume the remaining entities (e.g., solitary nonparasitic bile duct cysts, periportal cysts, biliary cystadenomas) and important differential diagnoses (e.g., abscesses, echinococcal cyst).
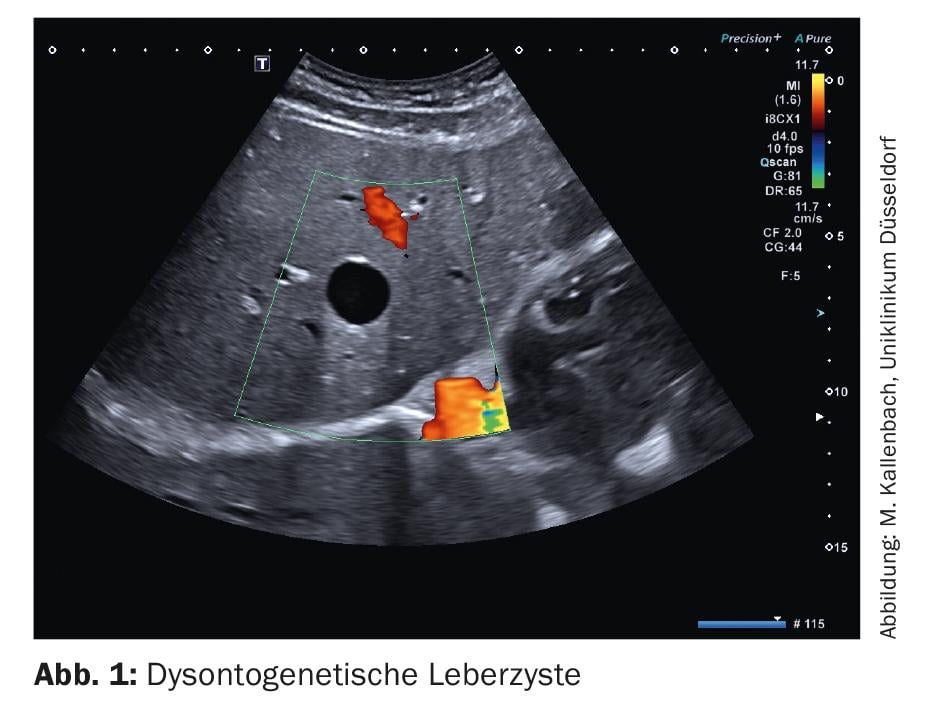
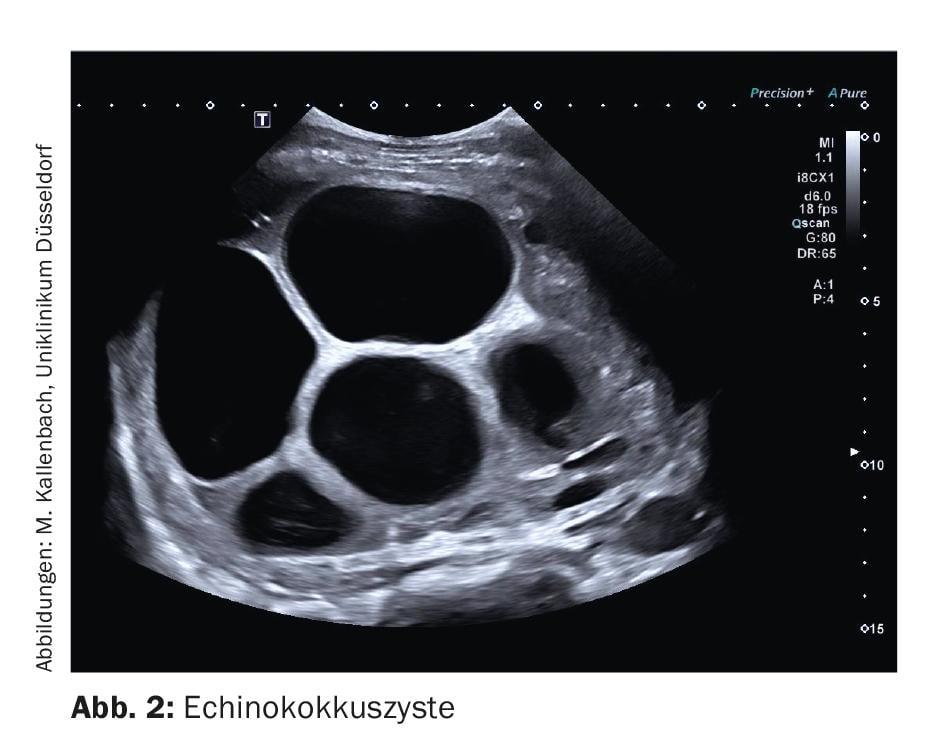
Dysontogenetic liver cysts are cavities filled with serous fluid and lined by single layer epithelium. Their diagnosis is the domain of gray-scale sonography. The most important cyst criteria here are: absence of echo, sharp border, delicate wall reflex, dorsal sound enhancement, cyst border shadow (Fig. 1). In duplex sonography, dysontogenetic cysts do not show vessels. If the cyst criteria are met with certainty, no further diagnostics are necessary. On the other hand, if the criteria are not taken seriously, misdiagnosis can easily occur. In particular, small vessel-rich low-echo space lesions (e.g., metastases) should be mentioned here, as these may appear anechoic if the image setting is suboptimal. As soon as cystic lesions show a strong echo-rich wall, a double contour, echogenic contents or solid wall portions, further clarification should be performed (Fig. 2). In this case, contrast-enhanced sonography may be helpful and may demonstrate perfused wall portions.
Solid liver lesions
Definite etiologic assignment of solid liver lesions is ultimately not possible without the use of contrast enhancers. However, based on typical appearances and a known frequency distribution, a sufficiently valid assignment can be made even without contrast medium, depending on the clinical context. It is also usually possible to assess the urgency of further clarification on the basis of the gray-scale image.
In addition to the solid liver lesions already listed above, other entities important in the context of differential diagnosis will be presented here.
1. hemangiomas are the most common benign liver neoplasms and are of mesenchymal origin. They consist of a convolute of blood vessels and little stroma. The numerous interfaces between the vessel lumina and vessel walls give rise to the mostly echoic appearance (Fig. 3). With increasing fatty degeneration of the liver parenchyma, it becomes more echoic and the difference in echogenicity between the hemangioma and the surrounding parenchyma becomes smaller. Especially in the presence of a fatty liver, a hemangioma can therefore be presented with low echo. Such hemangiomas are commonly referred to as “atypical.” Differentiation from other – mainly malignant – liver lesions is then not possible without the use of contrast enhancers.
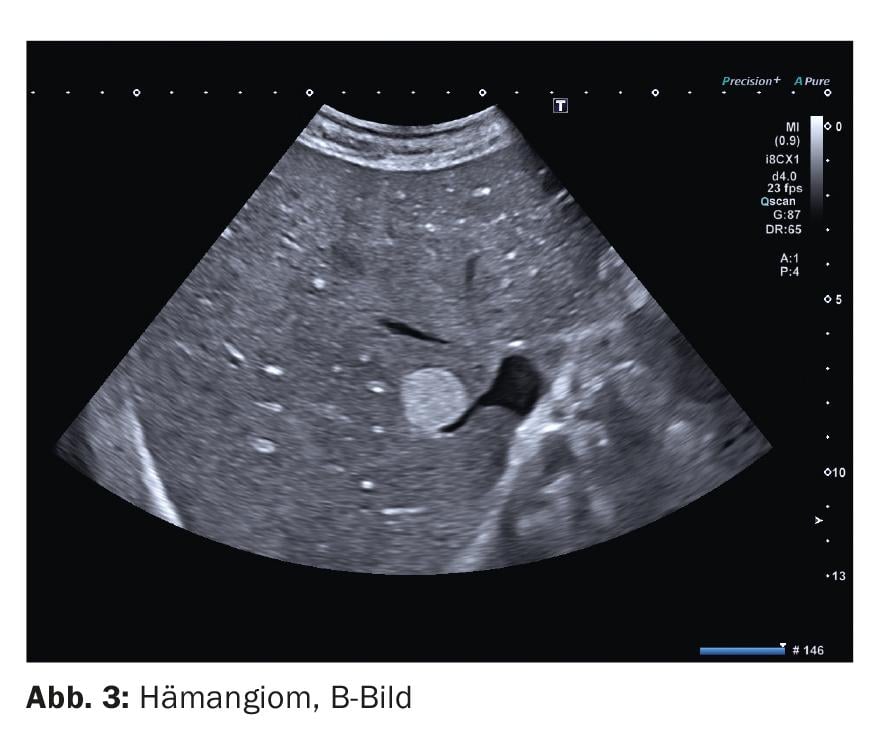
In duplex sonography, a feeding vessel is often identifiable. Vessels within the lesion are usually not depictable.
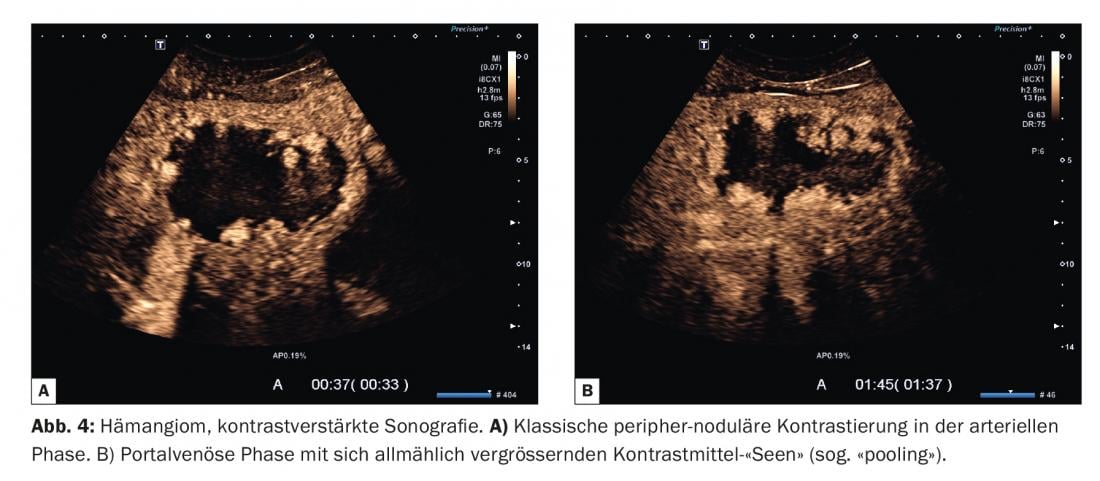
The contrast behavior of hemangiomas is also not uniform. A large group of hemangiomas shows the typical “peripheral nodular” contrast (Fig. 4) . Here, microbubbles in the form of nodules or contrast lakes accumulate either simultaneously with the surrounding parenchyma or prematurely in the marginal area of the lesion. Individual vessels are usually not recognizable. In the course of the investigation, an increase in the size of these lakes occurs, the so-called “pooling”. Finally, the contrast accumulations confluence and fill the lesion from the edge inward (centripetal) (Fig. 5).
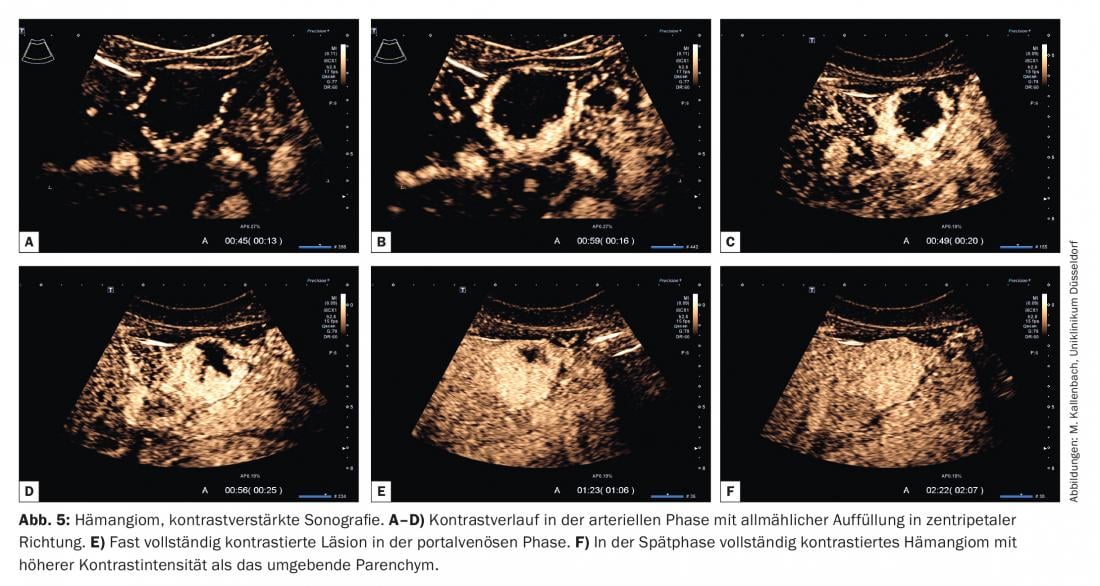
For this purpose, the term “iris phenomenon” was adopted from CT imaging. Filling may be complete or portions of the hemangioma may remain free of microbubbles due to sclerotherapy. The contrast intensity is typically higher than that of the surrounding parenchyma and is maintained for a long time (until the late phase). Few hemangiomas show focal inferior contrast in the portal venous or late phase – especially with long duration of sonication and associated artificial bubble destruction – and thus may be mistaken for malignant lesions. However, if peripheral nodular contrast was clearly detectable in the arterial phase, it is crucial for species diagnosis.
Hemangiomas with high blood flow are more difficult to diagnose. They are characterized by rapid contrast in the centripetal direction. Depending on the size of the hemangioma, the time required for complete filling is only a few seconds. Thus, assessment of the contrast pattern in the arterial phase is more difficult. As another complicating feature, some high blood flow hemangiomas lack the typical peripheral nodular pattern.
In these cases, a clear etiologic assignment will not succeed. A statement on the dignity can then only be derived on the basis of the behavior in the late phase.
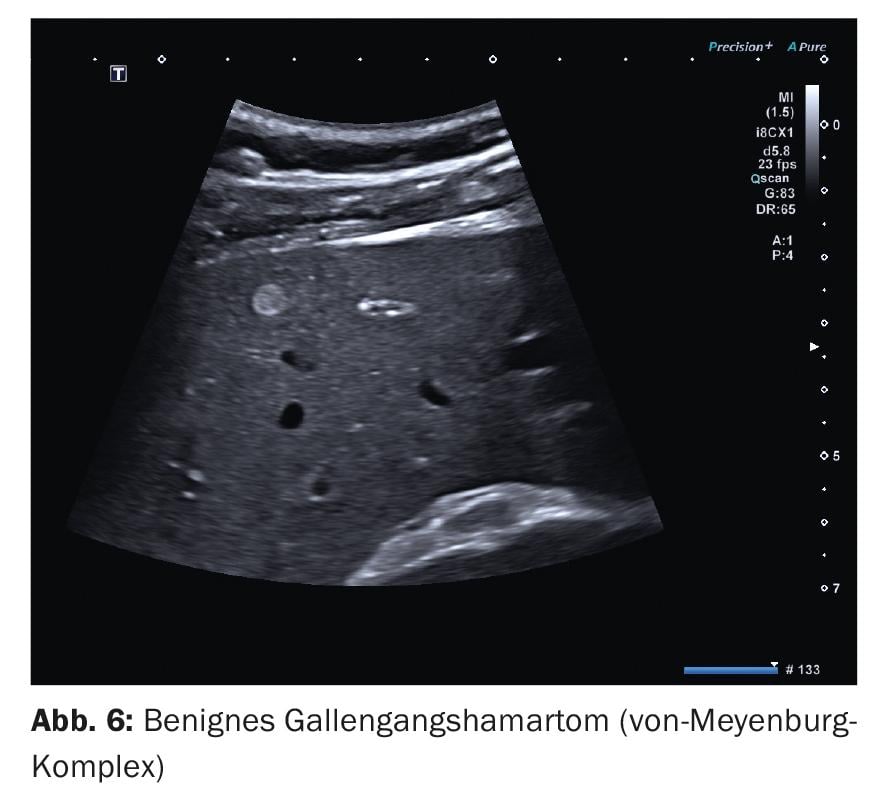
2. other echo-rich lesions are benign bile duct hamartomas, the so-called von Meyenburg complexes . They belong to ductal plate malformations and represent proliferations of bile ducts with cystic dilatations in a fibrous stroma [3]. The echo-rich lesions are usually spherical and small, i.e., the diameters are often less than 10 mm, only in isolated cases up to 20 mm (Fig. 6). No vessels can be delineated by duplex sonography. Assessment of contrast in the arterial phase is usually not useful because of its small size. In larger structures, there is usually no specific contrast behavior and no contrast behavior that deviates from the surrounding parenchyma. Important for differentiating small echo rich metastases is the lack of inferior contrast in the late phase.
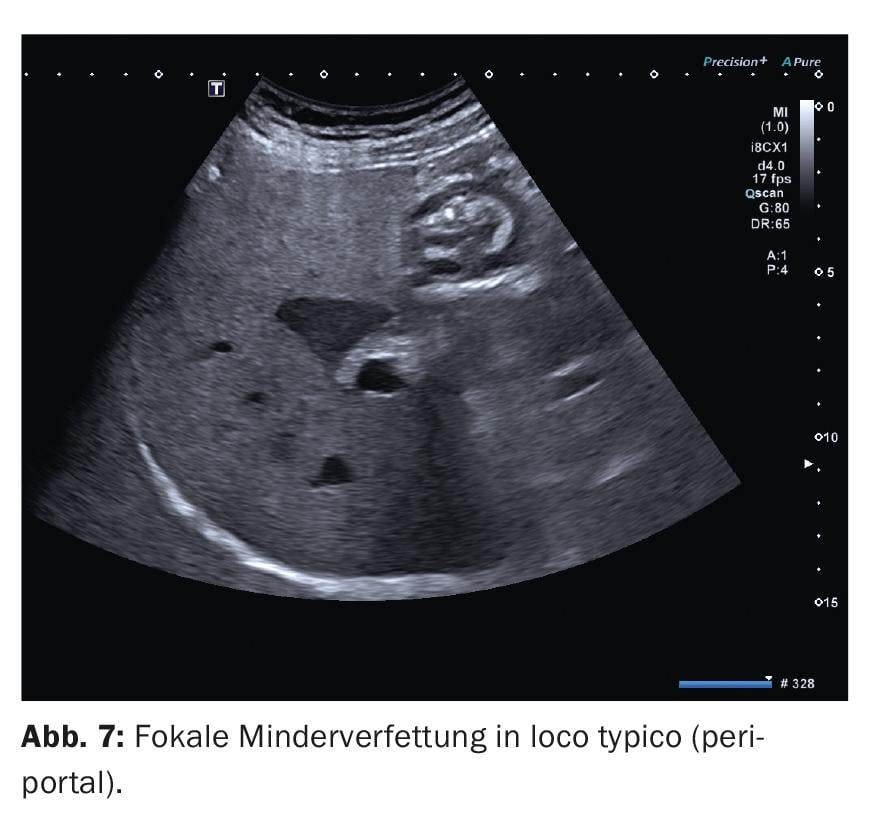
3. focal fatty disorders are usually irregularly circumscribed areas in the liver parenchyma where the hepatocytes have a different fat content. A reasonable suspicion of the presence of a focal fatty disorder can already be raised from the B-scan, provided they are encountered at the typical predilection sites: Focal multiple fatty lesions (echo-rich) are usually found ventral to the portal vein bifurcation in S IV and along the lig. falciforme. Focal reduced fat (echo-poor) in the gallbladder bed and also periportally (Fig. 7). The administration of contrast medium should be considered especially if the lesion is localized elsewhere and is not irregular or “map-like” limited, but rather spherical. Contrast will then show no difference from the surrounding parenchyma at all stages.
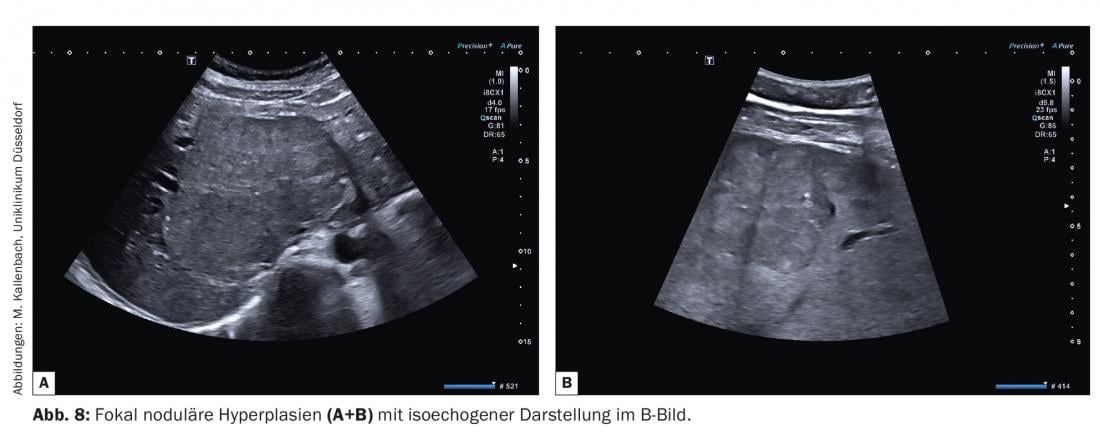
4. focal nodular hyperplasias (FNH) are tumor-like entities rather than neoplasms in the strict sense. They are the most common benign solid space lesions after hemangiomas and are clustered in young women (5-12 times more common than in men) [6]. The pathophysiologic basis is an arteriovenous (AV) fistula, which explains major contrast characteristics of these lesions. Hyperplasia of hepatocytes occurs. Therefore, the echogenicity of an FNH in a healthy liver usually differs little from that of the surrounding parenchyma (ca. 60% are isoechogenic, Fig. 8A and B) . Similar to the example of hemangiomas already explained, FNH in fatty liver may appear echo-deficient. Aging processes may increase the echogenicity of the FNH due to sclerosis, which also affects the contrast behavior.
An important feature in the B-scan is the frequently encountered lobulated contour [3,6], which can be regarded as a distinguishing criterion especially from mostly smooth-bordered adenomas. Other distinguishing marks are based on the vessel architecture. The classic variant of FNH shows a central artery that branches like a wheel spoke (angioarchitectural type I according to Wermke [3], Fig. 9). The artery and its branches are embedded in thick connective tissue septa. Under good acoustic conditions, these can already be guessed at in the B-scan as echo rich strands (central “scar”). If the typical vascular pattern can be visualized by duplex sonography, virtually no differential diagnoses are possible. Diagnostic confidence can be increased by contrast-enhanced sonography. In particular, if the vessel branching cannot be visualized in duplex or in case of an eccentric vessel branching (angioarchitecture type II according to Wermke [3]), it is necessary to perform KM ultrasonography.
The underlying AV malformation of FNH explains the main contrasting phenomena: due to low vascular resistance, contrasting of FNH typically starts before the surrounding parenchyma and proceeds rapidly: within a few seconds (2-3 s), even larger structures are completely filled with microbubbles (Fig. 10).
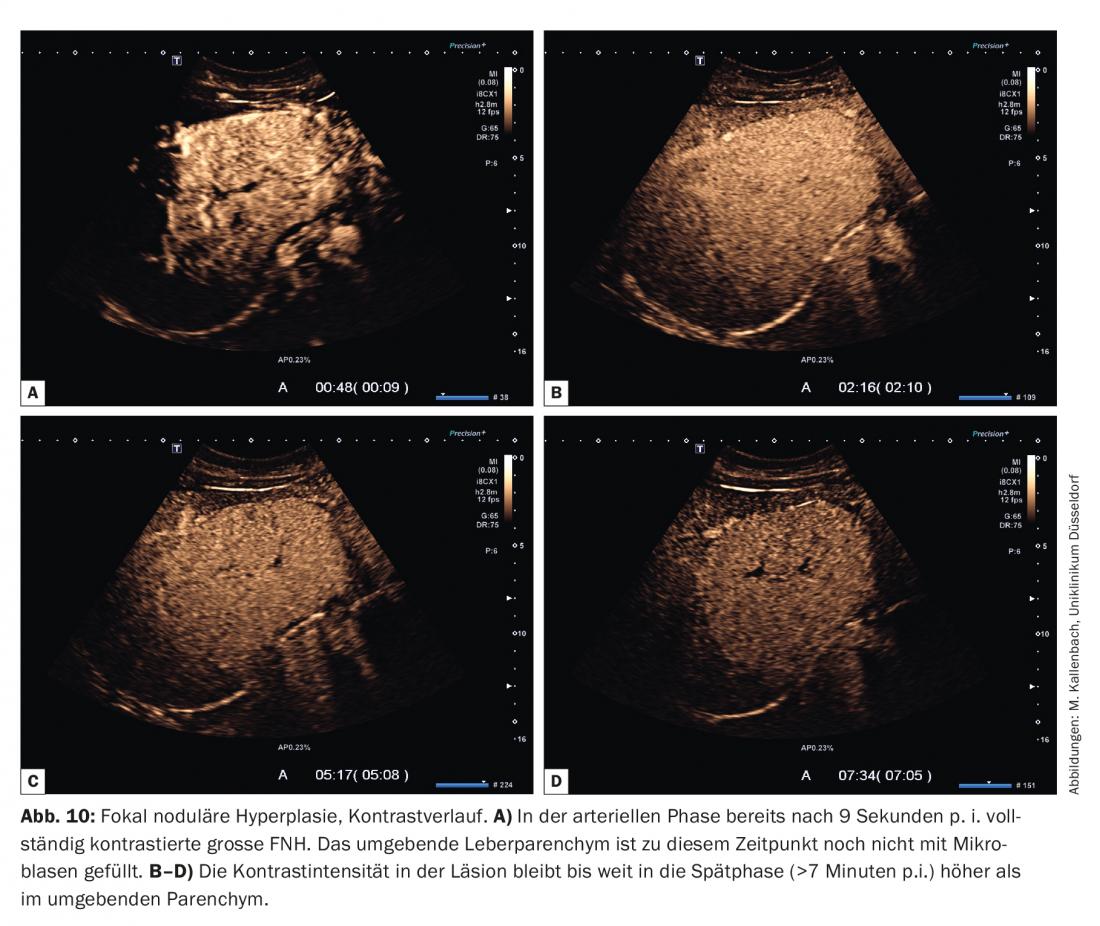
In the classic branching pattern, contrast begins in the center of the lesion and continues to the periphery (centrifugal). To assess the vascular architecture, the video clip of the arterial phase should be viewed frame by frame because of the rapid filling. It is also helpful here to perform (if necessary repeatedly) a bubble destruction in the arterial phase (so-called replenishment – or flash/burst maneuver). This allows the microbubble influx to be reassessed in the selected section plane.
In the portal venous and late phases, the contrast intensity of the FNH is at least as high as that of the surrounding parenchyma, in many cases even higher (Fig. 10). Mild focal underperfusion in the late phase is very rare and is observed mainly in FNH in advanced degenerative metamorphosis.
5. hepatocellular adenoma (HCA) is a rare neoplasm (prevalence about 0.001-0.004%) [2]. Similar to the FNH, they show a preference for the female sex (males:females = 1:10). In recent years, knowledge of their molecular pathology has grown significantly. Based on immunohistochemical markers, a subdivision into 4 subtypes was proposed a good 10 years ago [7]. The subtypes differ in terms of the rate of complica-tio-ns, such as hemorrhages and malignant degeneration. Therefore, the possibility of image morphologic differentiation of subtypes would be desirable for further management of patients with adenomas. This is all the more true because the risk of bleeding during puncture of adenomas is higher than for other tumors. The current valid EASL guidelines from 2016 [2] confirm MRI with liver-specific contrast agent (gadolinium) to have good accuracy in determining the subtype (80% d. F.), but recommend clinical management independent of the subtype. From this it is clear that currently establishing the correct diagnosis of “adenoma” and clinical risk factors are of greatest importance in the management of patients with HCA. Therefore, the subtypes and possible sonographic criteria for their differentiation will not be discussed further at this point.
According to EASL recommendations, all adenomas should be resected in men because they are at increased risk for malignant transformation. Women are advised lifestyle modification (abstaining from oral contraceptives, weight reduction) and follow-up after 6 months regardless of tumor size. Resection is recommended for tumor size >5 cm or relevant progression, otherwise continued observation.
Sonographically, hepatocellular adenomas may show variable echogenicity. In the healthy liver, they usually appear isoechogenic or low-echo, and in the fatty liver, they appear low-echo. Adenomas induced by contraceptive use often present as echo-rich. Typical of adenomas is a smooth contour and sometimes evidence of a fine echo-rich capsule. Duplex sonography often reveals vessels extending from the margin into the lesion (Fig. 11A and B) .
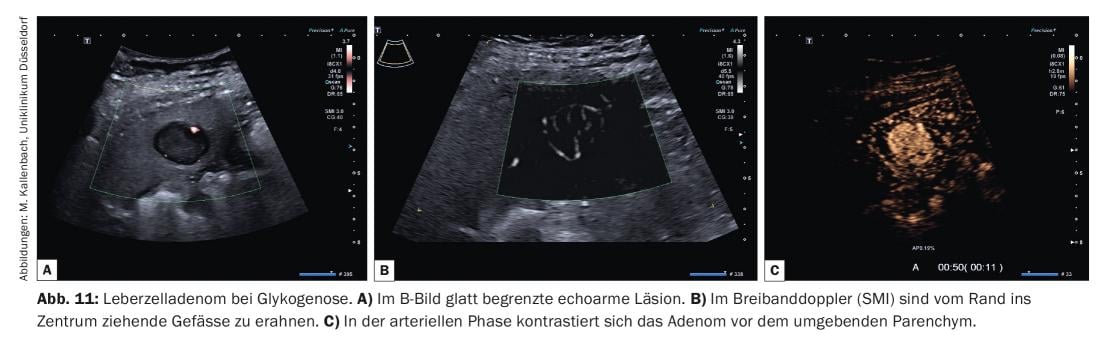
In KM ultrasonography, perfusion of adenomas in the arterial phase usually begins before the surrounding parenchyma (Fig. 11C). There is rapid and predominantly homogeneous contrast from the edge of the lesion toward the center (centripetal). The vessel architecture appears orderly and regular. Because intratumoral hemorrhage is not uncommon, portions of the tumor may be left out. In the portal venous phase, the tumor presents homogeneously perfused with contrast intensity at or slightly above that of the surrounding liver parenchyma. In the late phase, contrast intensity is maintained at a high level for a long time in some of the adenomas, while other adenomas show a slight reduction in contrast (“wash-out”).
In these cases, the differential diagnosis, especially to HCC, is difficult.
Sonography is the essential imaging modality for the evaluation of focal liver lesions. The use of ultrasound contrast media is standard today and allows a clear etiological assignment in many cases. If this is not possible, MRI (with liver-specific contrast agent) and finally biopsy are important complementary diagnostic procedures.
Take-Home Messages
- Sonography is indispensable in the diagnosis of liver lesions.
- Gray-scale sonography is primarily used to detect lesions (detection), while contrast-enhanced sonography is used to characterize them.
- If clear cyst criteria are present, no further diagnostics are required except for gray-scale ultrasonography. Here, sonography is superior to CT, especially for small lesions.
- Sharply circumscribed echo-rich liver lesions can be classified as hemangiomas in healthy individuals (no history of tumor, no chronic liver disease), especially when solitary and with a diameter <3 cm, on the basis of gray-scale and duplex sonography [2]. Von Meyenburg complexes may be considered as a differential diagnosis; the distinction is clinically irrelevant.
- Adipose disorders can already be diagnosed by gray-scale sonography if they have a typical location and shape.
- Isoechogenic hepatic space lesions with lobulated contour and a radially branching central vessel usually correspond to focal nodular hyperplasia (FNH) in young women. Contrast-enhanced sonography is very helpful for reliable differentiation of hepatocellular adenoma and malignant lesions.
Literature:
- Kaltenbach TE, Engler P, Kratzer W, et al: Prevalence of benign focal liver lesions: ultrasound investigation of 45,319 hospital patients. Abdominal radiology 2016; 41(1): 25-32.
- EASL Clinical Practice Guidelines on the management of benign liver tumours. Journal of hepatology 2016; 65(2): 386-398.
- Wermke W: Sonographic differential diagnosis – liver diseases. Cologne: Deutscher Ärzte-Verlag 2006.
- Strobel D, Seitz K, Blank W, et al: Tumor-specific vascularization pattern of liver metastasis, hepatocellular carcinoma, hemangioma and focal nodular hyperplasia in the differential diagnosis of 1,349 liver lesions in contrast-enhanced ultrasound (CEUS). Ultrasound Med 2009; 30(4): 376-382.
- Claudon M, Dietrich CF, Choi BI, et al: Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver–update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound in Medicine 2013; 34(1): 11-29.
- Fondis K: Studies on the long-term course of focal nodular hyperplasia 2009.
- Bioulac-Sage P, Rebouissou S, Thomas C, et al: Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology 2007; 46(3): 740-748.
FAMILY PRACTICE 2020: 15(11): 6-12