There are three main systems available for inhalation in children: Metered dose inhalers, dry powder inhalers and moist nebulizers. In addition, there are further developments of inhalers and systems with new features are coming onto the market.
There are essentially three different systems available for inhalation in children: Metered dose inhalers (pMDI), dry powder inhalers (DPI) and nebulizers. In addition, there are further developments of inhalers, such as the Autohaler® and the Respimat®, and systems with new features are coming onto the market, including inhalers with a control function. Each system has its advantages and disadvantages, and the inhalation system should first be selected according to age (Tab. 1) . Above all, the operation of the inhaler and the particle size generated must be age-appropriate. In addition, of course, the diagnosis must be taken into account, as well as individual parameters, such as the patient’s state of health and preferences, and reimbursement by health insurance companies.
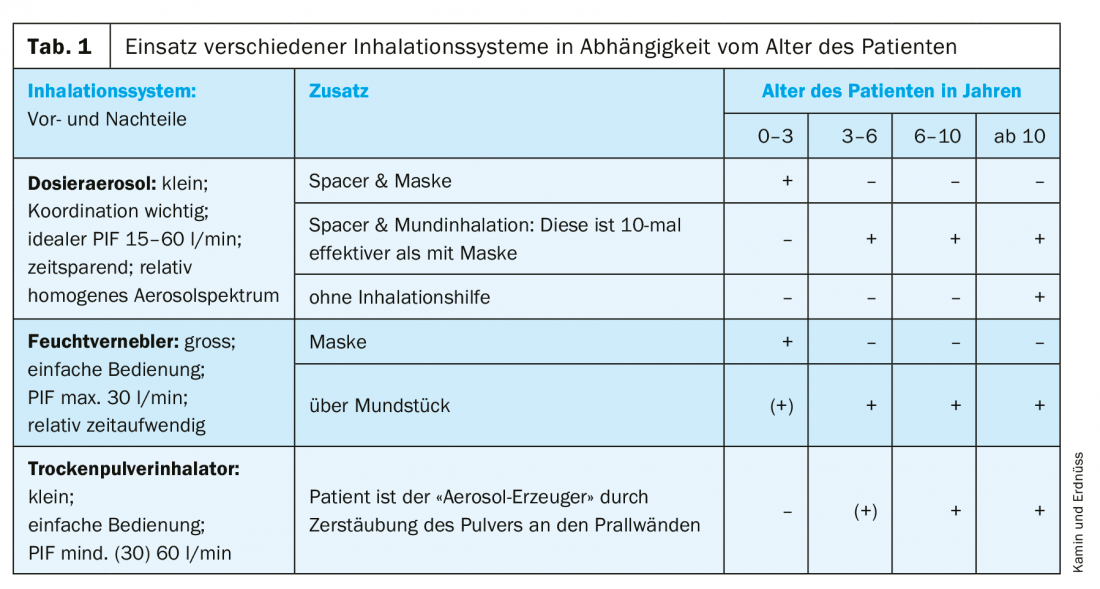
As shown in table 1, the youngest patients should preferably be supplied with jet or membrane nebulizers. Up to the age of 2, mask inhalation is advantageous because infants breathe predominantly through the nose and therefore cannot use a nebulizer by mouth properly. However, the mask must close 100% tightly over the patient’s mouth and nose, because even a small leak drastically reduces the amount of active ingredient inhaled to almost zero [14]. In this age group, it is also possible to use metered-dose inhalers with spacers, also with a mask, but they place somewhat greater demands on patient coordination. Nebulizer systems are still the easiest to use. It should be noted, however, that at this age the bronchial deposition achieved with these systems is no more than about 5% of the nominal dose [1,8].
From the age of 3, nebulizers and metered dose inhalers with spacers can also be used without a mask. Inhalation then takes place directly through the mouth, which is a great advantage because the active ingredient particles reach the bronchi directly without the detour via the nose. Oral inhalation is about ten times more effective for bronchial deposition than mask inhalation. It is important – if possible – to inhale slowly and evenly (resting breathing; max. 30 l/min). Therefore, peak inspiratory flow (PIF) is limited by an automatic control mechanism in some nebulizer models.
From school age, dry powder inhalers can then also be used. They are small and particularly easy to handle. Coordination of triggering the spray burst and inhalation is not necessary, since in them the deagglomeration of the active ingredient from the carrier molecule lactose occurs through the inhalation flow (PIF). However, it must be at least 30 l/min (better 60 l/min), and this is usually only achieved by older children.
Advantages and disadvantages of the different inhalation systems
Nebulizers are large and relatively time-consuming to use because the applied dose per breath is small. Depending on the inhaled volume, inhalation must be performed for up to ten minutes, and the PIF should not exceed 30 l/min. Mixing different inhalation solutions may save time, but care should be taken when doing so. The compatibility of the substances must be tested, otherwise incompatibilities or inefficacy of the active ingredients may result. As our studies show, certain incompatibilities occur when some substances are mixed, which, among other things, limit efficacy [13]. In contrast, no incompatibilities were found for other mixtures, such as Colistin CF® with hypertonic NaCl solution (Table 2); we are currently testing the compatibility of hypertonic saline with another antibiotic (aztreonam).
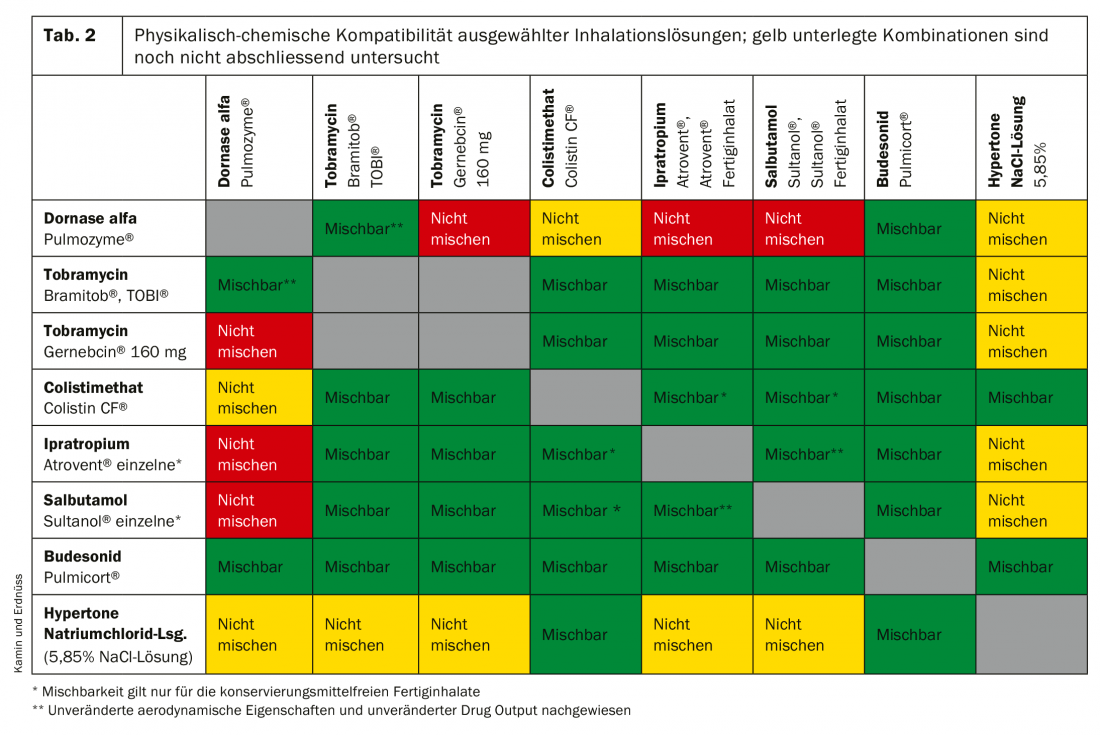
Furthermore, extensive in vitro investigations have shown that commercially available compressed air nebulizers generate very different aerosol spectra and that the amount of aerosol emitted is only of limited use for dose estimation. However, a relatively reliable value for device selection is the respirable lung dose (RDDR), which is calculated from the fine particle spectrum (FPF) and the delivered drug dose (DDR) [18]. However, the great advantage of nebulizers is and remains their ease of use (suitable for infants and young children, with mask if necessary) as well as the therapist’s ability to influence the aerosol spectrum via various baffle plates. In addition, special further developments of nebulizer systems can reduce the disadvantages described [15].
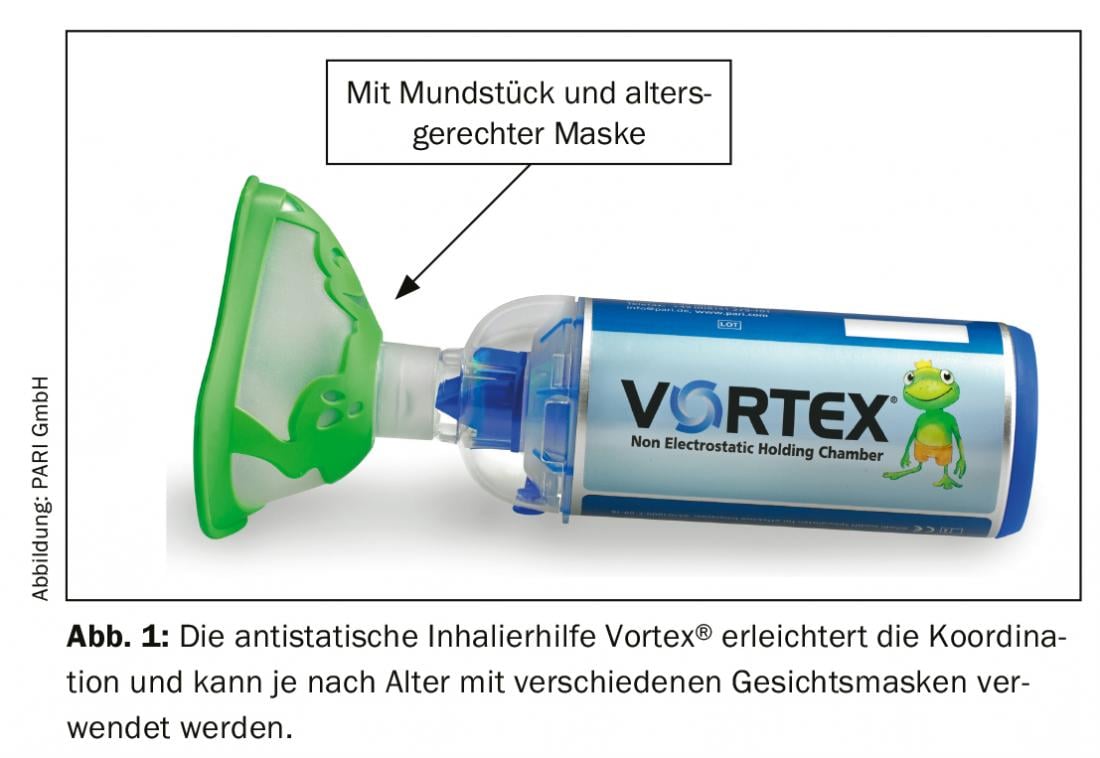
Metered dose inhalers are also constantly being improved [17] and can be used successfully with spacers in children as young as three years of age if the patient can produce a slow, steady PIF of 15-60 l/min. Spacers facilitate the coordination of release and inhalation, often a critical point in young patients. Compared to nebulizers, metered dose inhalers are conveniently small and can be used with significantly less time expenditure; the size of the aerosol particles also does not vary as much as with nebulizers. Among the propellant-gas-driven, CFC-free metered dose aerosols, so-called solution aerosols are particularly recommended for children. They produce smaller active ingredient particles overall, which find their way into the bronchial tubes more easily. Mask inhalation with metered dose inhalers can be performed up to 2 years of age in exactly the same way as with nebulizers, by placing a mask on the spacer. To reduce aerosol losses due to electrostatic forces, the spacer should be pretreated with flushing agent or have antistatic properties (Fig. 1).

One improvement was the Autohaler® (Fig. 2), an inhaler in which the spray is triggered by the breath. The Autohaler® can therefore also be used without spacers for children. A technical advancement was achieved with the Respimat® Soft Mist Inhaler (Fig. 3) . As a single-substance nozzle nebulizer, it combines features of both nebulizer and MDI: the aerosol cloud is triggered at the touch of a button, as with other MDIs, but coordination for quiet, timely inhalation is greatly facilitated by the slow, long aerosol cloud discharge (at 0.8 m/s for 1.5 s). Together with the high proportion of fine particles (<5 µm), this improves the deposition of the active ingredient in the lungs, so that the dose can be reduced compared to other devices – with the same effectiveness [3,5]. With a spacer and, if necessary, a mask, the Respimat® can also be used successfully with small children. Unfortunately, there are few possible applications in pediatrics, as the substances available for the innovative device are almost exclusively suitable for the therapy of COPD.

Dry powder inhalers are also available in a pocket format, but are only suitable for children in school age due to the high PIF required of at least 30 l/min (Table 1). In DPIs, the release and deagglomeration of the drug is triggered only by the inhalation flow; the higher it is, the more effective the release. However, this positive effect is countered by impaction; this refers to the deposition of drug particles in the upper airways; impaction also increases with increasing respiratory flow, and this is the case for all inhalation systems [7,9]. Overall, DPIs place low demands on coordination: unlike pMDIs, for example, triggering too early or too late here cannot reduce the amount of drug inhaled. According to the most recent studies, the pressure drop during inspiration is crucial for an adequate lung dose: it should be at least 1 kPa (equivalent to 10 cm water column) [7]. Patients with lactose intolerance often have concerns about DPIs because the active ingredient is almost always bound to lactose; however, due to the low doses, no clinical symptoms are expected.
The right diagnosis
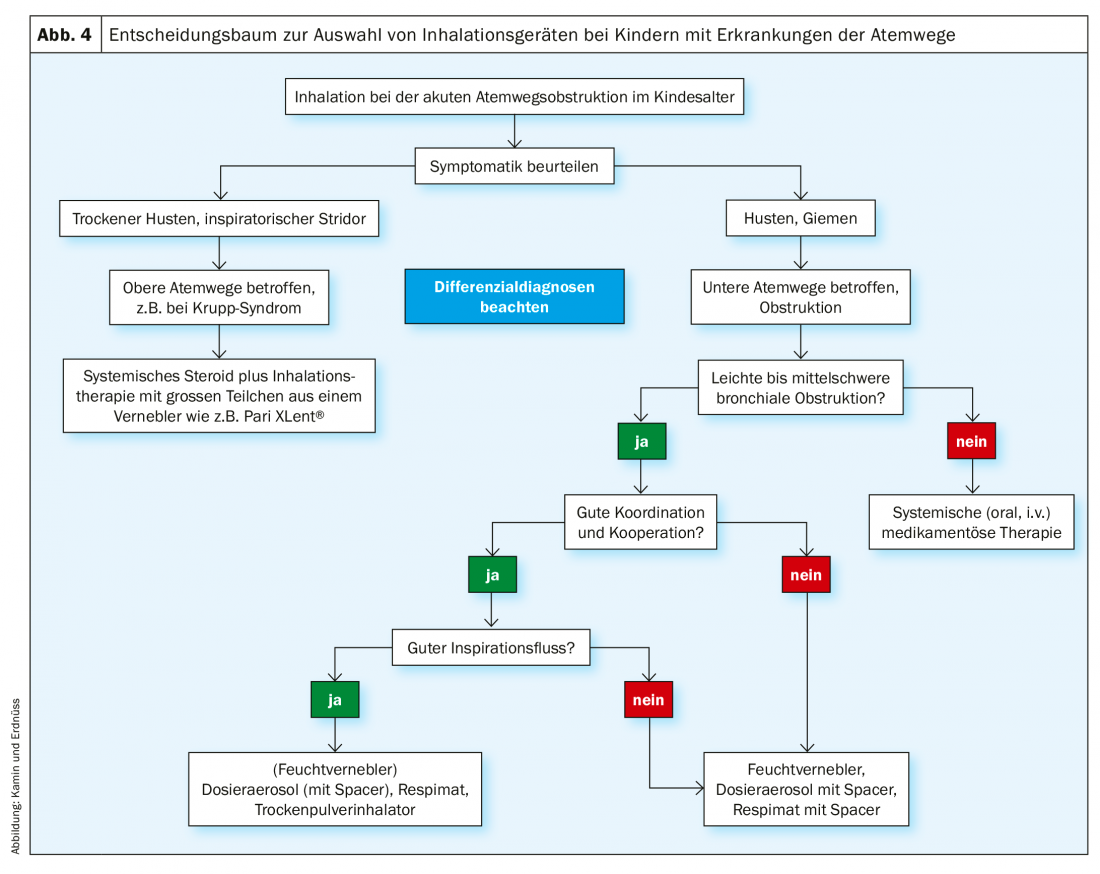
In addition to age, the diagnosis naturally also plays a role in the selection of the inhalation system. For example, if the upper respiratory tract is affected, such as sinusitis or croup syndrome, a moist nebulizer with appropriate particle spectrum or technique is the system of choice, regardless of the patient’s age. Special models (Figs. 6 and 7) can be used to generate the pulsating aerosol or sufficiently large active ingredient particles required for successful therapy. If, on the other hand, the lower airways are to be treated, the patient’s handling and inspiratory flow play an important role in the selection of the inhaler (Fig. 4) . The specific handling of the inhalation system should be well trained before the start of therapy.
Treatment of the lower respiratory tract
Inhalation therapy is regularly used in children with bronchial obstructive diseases of the lower respiratory tract (e.g. asthma, bronchitis, ciliary dyskinesia, cystic fibrosis, pneumonia). Typical clinical symptoms include wheezing or coughing. In addition, allergic and infection-associated bronchial obstruction are essentially characterized by three pathological changes in the airways: Hypersecretion, mucosal edema, and bronchial smooth muscle contraction. (Fig. 4). Before initiating inhalation therapy, the therapist should be sure of the diagnosis, with different differential diagnoses to consider according to age (Tab.3).
Child bronchi need small particles
For inhalation therapy in children and adolescents, one of the factors that should be taken into account is that their airways have a much smaller diameter than those of adults [10]. Inflammation, mucus formation and contraction of the bronchial smooth muscle can cause them to narrow even further (Fig. 5), so that the size of the active ingredient particles generated by the inhaler (the so-called fine particle spectrum) plays an important role. Consequently, children with lower respiratory tract diseases need a fine particle spectrum with particularly small diameters. If the active ingredient particles are too large, a large part of the dose already remains in the mouth and throat and can then also cause unwanted side effects there [2]. In principle, for children, as many of the inhaled particles as possible should have a diameter of ≤3 µm (for comparison, a red blood cell has a diameter of 6-7 µm). However, even with an age-appropriate particle spectrum, the amount of active ingredient that reaches the lungs during inhalation increases with age, but without any change in the effective dose relative to body weight [19]. Therefore, a significantly higher individual dose per kg body weight is prescribed for young children than for adolescents [20].
Treatment of the upper respiratory tract
Infants repeatedly present with dry cough, hoarseness, and inspiratory stridor. These are typical symptoms of croup syndrome. It is estimated that approximately 5% of children between the ages of 3 and 36 months are affected each year [4,6]. In addition to the application of systemic steroids, treatment with inhaled adrenaline (epinephrine) promises good results here [21], provided that the appropriate application system is selected. This is because aerosols can be significantly larger for upper respiratory tract therapy than for inhaled treatment of bronchitis, for example. A particle spectrum between 7 and 9 µm is favorable here, so that the PARI XLent® , for example, can be used (Fig. 6). Special inhalation systems are also available for other diseases of the upper respiratory tract (e.g. rhinitis, sinusitis, laryngitis, pharyngitis), e.g. the PARI Sinus® for sinusitis (Fig. 7).
Good cooperation and correct technique = successful therapy
In general, patient/family cooperation and adherence are the most important factors for successful inhaled therapy [11]. In addition, inhalation technique should first be well trained and then regularly reviewed [16], because only about one-third of children can implement the correct use of inhalation systems based on verbal explanations alone. For example, a quiet and deep breathing maneuver is generally recommended for metered dose inhalers, while a vigorous breathing maneuver is required from the start for powder inhalers. Incorrect inhalation technique may result in the administered drugs not reaching the lungs but being deposited extrathoracically by impaction; then, in any case, the systemic effect of the drug dose is too small to control the symptoms and, in the case of steroids, for example, there may be undesirable side effects in the mouth and throat.
Therefore, the simultaneous use of different inhalation devices for maintenance and emergency therapy is not recommended, as patients are often unable to correctly perform the devices’ fundamentally different inhalation maneuvers [12]. Thus, lack of therapeutic success is often due to incorrect inhalation technique. But also the correctness of the diagnosis, the type of drug or too low a dosage must be considered. If there is no improvement after 4-8 weeks of treatment, the patient or parents should first be asked whether inhalation is being performed regularly and correctly. Inhalation should also be demonstrated and follow-up training provided if necessary.
Take-Home Messages
- Inhalation therapy can be used successfully for both upper and lower respiratory tract diseases, although age-appropriate differential diagnoses must be considered before starting therapy.
- Correct technique/handling and good patient adherence are crucial for therapeutic success.
- The selection of the appropriate inhalation system is first based on age, then the fine particle spectrum of the inhaler and individual parameters of the patient (including health status, preferences) must be taken into account.
- The systems available are metered dose inhalers, dry powder inhalers, and wet nebulizers.
- Ideally, maintenance and emergency therapy should be performed with similar inhalation systems.
- Inhalation via the mouthpiece is much more suitable for therapy of the lower respiratory tract than inhalation via the mask.
Literature:
- Amirav I, Balanov I, Gorenberg M, et al: Beta-agonist aerosol distribution in respiratory syncytial virus bronchiolitis in infants. J Nucl Med 2002; 43(4): 487-491.
- Amirav I, Newhouse MT: Deposition of small particles in the developing lung. Paediatr Respir Rev 2012; 13(2): 73-78.
- Asakura Y, Nishimura N, Maezawa K, et al: Effect of switching tiotropium HandiHaler® to Respimat® Soft Mist™ Inhaler in patients with COPD: The difference of adverse events and usability between inhaler devices. J Aerosol Med Pulmon Drug Deliv 2013; 26(1): 41-45.
- Bjornson CL, Johnson DW: Croup in children. CMAJ 2013; 185(15): 1317-1323.
- Brand P, Hederer B, Austen G, et al: Higher lung deposition with Respimat Soft Mist inhaler than HFA-MDI in COPD patients with poor technique. Int J Chron Obstruct Pulmon Dis 2008; 3: 763-770.
- Cherry JD. Croup. N Engl J Med 2008; 358: 384-391.
- Clark AR, Weers JG, Dhand R: The Confusing World of Dry Powder Inhalers: It Is All About Inspiratory Pressures, Not Inspiratory Flow Rates. J Aerosol Med Pulm Drug Deliv 2019; Epub ahead of print Oct 31st; doi: 10.1089/jamp.2019.1556.
- Fok TF, Monkman S, Dolovich M, et al: Efficiency of aerosol medication delivery from a metered dose inhaler versus jet nebulizer in infants with bronchopulmonary dysplasia. Pediatr Pulmonol 1996; 21(5): 301-309.
- Janssens HM, Krijgsman A, Verbraak TF, et al: Determining factors of aerosol deposition for four pMDI-spacer combinations in an infant upper airway model. J Aerosol Med 2004; 17(1): 51-61.
- Janssens HM, Tiddens HA: Aerosol therapy: the special needs of young children. Paediatr Respir Rev 2006; 7 Suppl 1: S83-85.
- Kamin W, Genz T, Roeder S, et al: The inhalation manager: a new computer-based device to assess inhalation technique and drug delivery to the patient. J Aerosol Med 2003; 16(1): 21-29.
- Kamin W, Kreplin A: Training of the inhalation maneuver in children with bronchial asthma using visual feedback. Pulmonology 2007; 61(3): 150-156.
- Kamin W, Erdnüss F, Krämer I: Inhalation solutions – which one are allowed to be mixed? Physico-chemical compatibility of drug solutions in nebulizers. Update 2013. J Cyst Fibros. 2014; 13(3): 243-250.
- Lin HL, Wan GH, Chen YH, et al: Influence of nebulizer type with different pediatric aerosol masks on drug deposition in a model of a spontaneously breathing small child. Respir Care 2012; 57(11): 1894-1900.
- Longest W, Spence B, Hindle M: Devices for Improved Delivery of Nebulized Pharmaceutical Aerosols to the Lungs. J Aerosol Med Pulm Drug Deliv 2019; 32(5): 317-339.
- Pearce L: How to teach inhaler technique. Nurs Times 2011; 107(8): 16-17.
- Roche N, Dekhuijzen PN: The Evolution of Pressurized Metered-Dose Inhalers from Early to Modern Devices. J Aerosol Med Pulm Drug Deliv 2016; 29(4): 311-327.
- Walz-Jung H, Kamin W, Krämer I: In vitro investigations of aerosol characteristics of different compressed air nebulizers for children in simulation models with salbutamol. Hospital Pharmacy 2018; 39: 379-389.
- Wildhaber JH, Janssens HM, Pierart F, et al: Highpercentage lung delivery in children from detergent-treated spacers. Pediatr Pulmonol 2000; 29: 389-393.
- Wildhaber J, Kamin W (eds.): Inhalation therapy in childhood and adolescence. 2nd ed. 2010. UNI-MED Verlag aG. Bremen
- Zoorob R, Sidani M, Murray J: Croup: an overview. Am Fam Physician 2011; 83(9): 1067-1073.
InFo PNEUMOLOGY & ALLERGOLOGY 2019; 1(3): 12-17.











