Bronchial carcinomas are the leading cause of cancer-related deaths worldwide. With the development of immune checkpoint inhibitors, the therapeutic landscape has changed significantly.
Bronchial carcinoma diagnosis and therapy is a rapidly changing field and of considerable clinical interest due to the high incidence and mortality of these tumor diseases worldwide. The rapid increase in knowledge in molecular biology and immunology and advances in molecular diagnostics are driving the development of personalized tumor treatment (“precision medicine”). This development is far from complete.
In this article, we focus on recent innovations in the treatment of bronchial carcinoma with emphasis on systems therapy of advanced tumor stages in the first line of therapy. These are treatment approaches that we are currently pursuing at our institution. We make no claim to completeness or absolute validity.
Basics
Bronchial carcinomas had the highest incidence (11.6%) and the highest mortality (18.4%) of all tumors worldwide in 2018, on par with breast tumors [1]. In the period 2011 to 2015, bronchial carcinomas ranked third in Switzerland in terms of incidence (approximately 4300 new cases per year), and first in terms of cancer-related causes of death [2]. The 5-year relative survival rate for lung cancer in Switzerland was 15% for men and 19% for women, respectively [3]. Tobacco smoking continues to be the leading cause of lung cancer [4]. Approximately 20% of bronchial carcinomas occur in “never smokers” (<100 cigarettes total). The main reason for this is exposure to inhalative noxae (e.g. radon, asbestos, tar vapor) [5].
Small cell lung carcinoma
In 10-15% of newly diagnosed bronchial carcinomas are small cell lung cancers (SCLC). Therapeutically relevant is the distinction between limited disease LD (stage I-III) and extensive disease ED (stage IV). The latter is much more common due to aggressive tumor biology. The median overall survival (OS) is 15-20 months for LD-SCLC and 8-13 months for ED-SCLC [6]. Most SCLC have inactivating mutations of tumor suppressor genes TP53 and RB1, while “treatable” mutations of receptor tyrosine kinases are usually absent (unlike non-small cell lung cancer, NSCLC). Current stage-dependent therapeutic standards can be summarized as follows:
Stage I: In addition to surgery, adjuvant platinum-based chemotherapy in stage I confers an OS benefit (66.0 vs. 42.1 months) [7].
Stage II, III: Surgery plus chemotherapy is an option in stage II; alternatively, surgery may be omitted and definitive radio-chemotherapy may be given. This is also the treatment of choice in stage III and should – if possible – be performed simultaneously [8,9].
Stage IV: Palliative systemic therapy is the treatment of choice for ED-SCLC. Despite an initially very good response to therapy, the median overall survival is only 10 months. Standard protocols are cisplatin and etoposide or carboplatin and etoposide [10]. The protocols differ mainly in terms of side effects, less so in terms of tumor effects [11]. Radiation of thoracic residual tumor is not a standard, but an individual option in case of very good therapeutic response to chemotherapy as well as complete remission of extrathoracic metastases [12].
Atezolizumab, a programmed death ligand 1 (PD-L1) inhibitor (immune checkpoint inhibitor, ICI), combined with chemotherapy and subsequently used as maintenance therapy, confers a survival benefit in the first line of therapy compared with chemotherapy alone (12.3 vs. 10.3 months, HR 0.7, 95% CI 0.54-0.91), as demonstrated in the phase III IMpower133 study [13]. The phase III CASPIAN trial with durvalumab and chemotherapy was also positive, the manufacturer recently reported [14]. Thus, ED-SCLC now has two new approaches to combined chemo-immunotherapy, assuming the compounds are still approved by drug authorities for this indication.
Although prophylactic whole-brain irradiation (PCI) is considered “standard” in many guidelines in stage II-IV after a good response to chemotherapy [15,16]. However, a Japanese study challenges this standard, showing no survival benefit for PCI compared with magnetic resonance imaging cranial controls alone [17]. These data already seem to be leading to a partial departure from the previous standard in clinical practice [18]. The impact of immunotherapy on the indication for PCI is still unclear.
Many patients with SCLC experience recurrence or progression within 6-12 months. The reason for this is probably the presence of cisplatin-resistant “cancer stem cells” [19]. Further treatment depends on the patient’s condition, the extent of tumor recurrence, prior therapy, and the treatment-free interval [15–17]. If a refractory situation or platinum-resistant relapse is present, topotecan is superior to supportive care alone [20] and equivalent to multiple therapy (cyclophosphamide, doxorubicin, and vincristine) in terms of overall response rate (ORR), progression-free survival (PFS), and OS, but with better tolerability [21]. Intravenously and perorally administered topotecan are roughly equivalent in terms of efficacy and tolerability [22]. Patients in good general health (ECOG 0-2) with a “platinum-sensitive recurrence” can be treated again with cisplatin/carboplatin and etoposide [23].
In our opinion, the use of immune checkpoint inhibitors after failure of chemotherapy is not (yet) a standard of care today. The best data to date are for pembrolizumab (in PDL1-positive tumors) [24] and for nivolumab [25].
Non-small cell lung cancer
Non-small cell lung carcinoma (NSCLC, approximately 85-90% of all bronchial carcinomas) is a clinical collective term for adenocarcinomas (50-60%), squamous cell carcinomas (20-25%), large cell carcinomas, adenosquamous carcinomas, neuroendocrine carcinomas (of which small cell lung carcinoma is excluded), and sarcomatoid carcinomas.
Stage I/II: Therapy of choice in stage I and II is radical surgery according to oncological standards (usually a lobectomy with systematic mediastinal lymph node dissection). From a tumor diameter of 4 cm, adjuvant chemotherapy with 4 cycles of a platinum-containing combination is indicated. If there are contraindications to a surgical procedure, stereotactic radiation is an alternative.
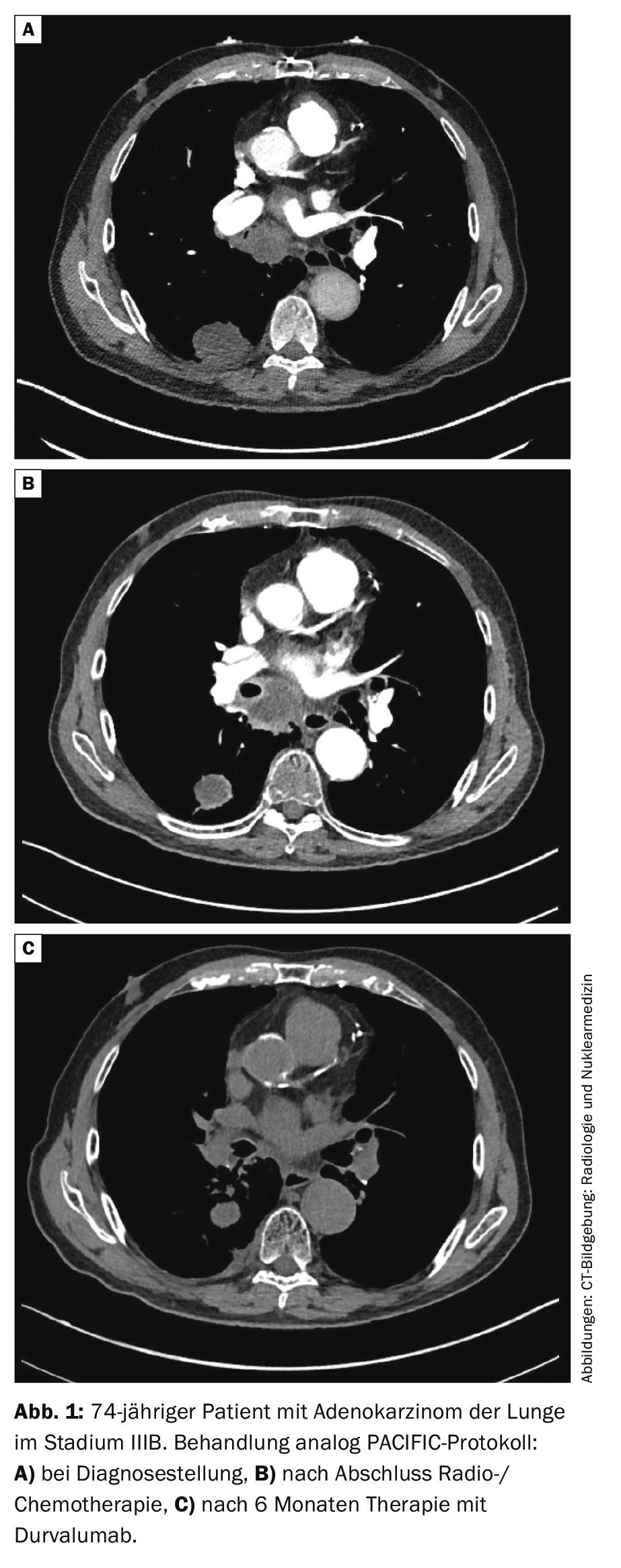
Stage III: For resectable tumors in stage III, chemotherapy is indicated in addition to surgery. This can be done before (neoadjuvant) or after (adjuvant) surgery. The benefit of additional radiotherapy (“trimodal therapy”) is controversial. The Swiss phase III SAKK16/00 trial was negative regarding preoperative irradiation [26]. Postoperative radiotherapy (PORT) showed no clear benefit in a retrospective meta-analysis [27], data from the LungART randomized trial are pending. Unresectable stage III NSCLC is radio-chemotherapied followed by one year of “consolidative” immunotherapy with the PD-L1 antibody durvalumab. This is based on the prospective randomized phase III PACIFIC trial [28, 29]. In the study, 2-year survival was 66.3% with durvalumab and 55.6% with placebo (HR 0.68, 99.73% CI 0.47-0.997, p=0.0025). Median PFS was significantly prolonged with durvalumab, compared to placebo (17.2 versus 5.6 months, HR 0.51, 95% CI 0.41-0.63, p<0.001). Therefore, consolidation with durvalumab is now considered the standard of care (Fig. 1).
Stage IV: Apart from so-called “oligometastatic” stages, where surgery and radiotherapy can be used with curative intent, most stage IV NSCLC is treated with palliative intent using drugs alone. A distinction is made between chemotherapy, targeted therapy and immunotherapy. The choice of therapy depends on the histologic subtype, the presence of driver mutations (in nonsquamous tumors), and the PD-L1 expression of the tumor. Molecular pathological workup is the basis for optimal therapy (Fig. 2).
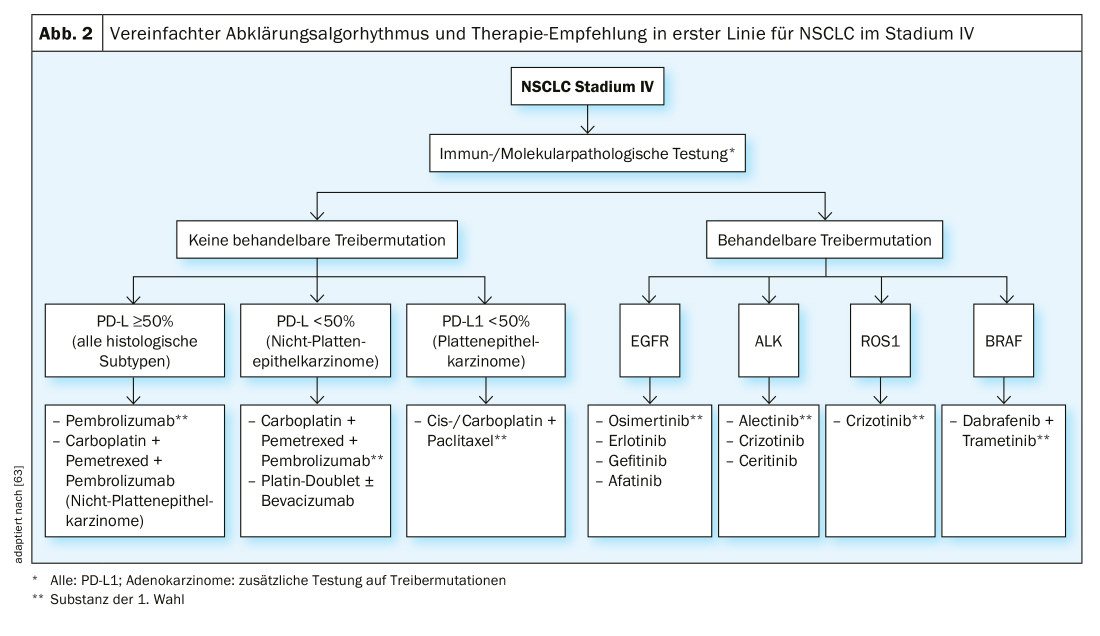
If no treatable “driver mutations” are present, treatment is primarily based on PD-L1 expression status. For NSCLC (regardless of histologic subtype) with PD-L1 expression of ≥50%, treatment with the PD-L1 inhibitor pembrolizumab is superior to conventional chemotherapy in terms of OS and PFS according to the KEYNOTE 024 study [30]. For adenocarcinomas of the lung with PD-L1 expression of ≤ 50%, a combination of chemotherapy and pembrolizumab is preferable to chemotherapy alone, analogous to the KEYNOTE 189 study [31]. The treatment response rate was 48% in the study with the addition of pembrolizumab and 19% without pembrolizumab. The 12-month survival rate was 69% for pembrolizumab + chemotherapy and 49% for the chemotherapy alone group. An alternative is the PD-L1 antibody atezolizumab in combination with carboplatin, paclitaxel, and bevacizumab, based on the results of the IMpower130 and -150 trials [32,33].
For squamous cell carcinoma with PD-L1 expression of ≤50%, a combination of pembrolizumab with chemotherapy is associated with OS and PFS benefit according to the KEYNOTE 407 trial [34].
If no immune checkpoint inhibitor was used in the first line of therapy, their use is indicated in the second line. The use of nivolumab is associated with a statistically significant ORR, OS, and PFS benefit, regardless of PD-L1 expression on tumor cells, compared with docetaxel [35].
Second-line treatment with atezolizumab was compared against docetaxel; regardless of NSCLC histology, an OS benefit was found in favor of atezolizumab [36,37]. Pembrolizumab was evaluated for tumors with PD-L1 expression of at least 1%, showing a significant OS benefit against docetaxel [38]. Treatment with immune checkpoint inhibitors should be given as long as a response is demonstrated or as long as the patient tolerates the treatment without the occurrence of severe side effects.
Driver Mutations: In a significant number of NSCLC, a mutation of a protooncogene to an oncogene encoding an activating receptor tyrosine kinase (RTK) is found. This mutation causes increased cell proliferation, resistance to apoptosis, angiogenesis and metastasis. Such mutations are called driver mutations. These are usually somatic mutations rather than germline mutations [39]. These findings led to the development of oral tyrosine kinase inhibitors (TKIs), whose effect is based on a more or less selective inhibition of the corresponding signal transduction pathway. Taking into account the pre-test probability, testing for the presence of mutations is currently mandatory for stage IV non-squamous NSCLC, but not for squamous cell carcinoma and SCLC. Due to time constraints, we test in two steps at our institution. The first step includes rapid tests for EGFR, ALK, ROS1, KRAS and BRAF. If negative, we test HER2, MET, RET, NTRK1-3 and other markers in a second step. Following, we address the targeted treatment of stage IV NSCLC with classic driver mutations.
EGFR: Activating mutations of the protooncogene “epidermal growth factor receptor” (EGFR) are found in 13-15% of pulmonary adenocarcinomas in Central Europe, clustered in non-smokers (prevalence over 50% depending on study) [40]. Approximately 90% of cases are either an exon 19 deletion or an exon 21 L858R point mutation [41]. Standard therapy in the first line is an EGFR TKI. This therapy, compared with platinum-based chemotherapy, is associated with a statistically significant PFS benefit [42]. Available TKIs include gefitinib and erlotinib (first generation), afatinib and dacomtinib (second generation), and osimertinib (third generation). Under first-generation EGFR TKIs, treatment resistance occurs on average after 10-14 months, in 50-60% of cases due to a second EGFR mutation (T790M) [43]. Osimertinib, a TKI with good CNS availability and efficacy in the presence of an EGFR T790M mutation, was evaluated against gefitinib and erlotinib in the FLAURA trial. Data published in 2017 demonstrated a statistically significant PFS benefit in favor of osimertinib (18.9 months with osimertinib versus 10.2 months with gefitinib and erlotinib, respectively, (HR 0.46; 95% CI 0.37-0.57; p<0.001) [44]. Osimertinib has been shown to have better efficacy against brain metastases and better tolerability. The latest data from the FLAURA trial, which will be presented in September at the 2019 ESMO Annual Congress, demonstrate for the first time a statistically significant survival benefit of osimertinib according to [45]. Thus, osimertinib will definitely establish itself as first-line therapy for all patients with EGFR-mutated NSCLC, regardless of T790M or brain metastases. The combination of an EGFR TKI with chemotherapy primarily in EGFR-mutated NSCLC is not currently considered standard of care, but has been investigated in several trials. The Japanese phase III NEJ009 trial compared gefitinib with gefitinib + chemotherapy (carboplatin and pemetrexed), showing a statistically significant PFS benefit (21 vs. 11 months; HR 0.49, 95% CI 0.39-0.63) and OS benefit (52 vs. 39 months; HR 0.70, 95% CI 0.52-0.93), but accompanied by a higher rate of grade 3 Toxicity (65% vs. 31%) [46]. Further studies (with erlotinib and gefitinib) confirm these results. The combination of the EGFR TKI erlotinib with the VEGFR antibody bevacizumab produced a PFS but not OS benefit compared with erlotinib monotherapy [47].
ALK: Translocations of the anaplastic lymphoma kinase (ALK) proto-oncogene are found in 3-5% of pulmonary adenocarcinomas [48], again clustered in nonsmokers [49]. Available ALK-TKIs are crizotinib, ceritinib, alectinib, brigatinib, and lorlatininb. The first-line agent of choice is alectinib, due to its good CNS mobility and superiority to crizotinib in the ALEX trial [50,51]. The choice of ALK-TKIs in additional lineages is based particularly on availability, brain involvement, and resistance mutations [52].
ROS1: Translocations of ROS proto-oncogene 1 (ROS1) are found in 1-2% of pulmonary adenocarcinomas, again clustered in nonsmokers. Due to the structural similarity of the ALK and ROS1 kinases, many ALK inhibitors other than alectinib are also useful for inhibiting ROS1. Treatment of ROS1 mutated lung tumors with crizotinib is the therapy of choice [53]. Lorlatinib showed very good efficacy in a phase II study, especially in resistance to crizotinib [54]. Ceritinib, entrectinib, and repotrectinib are other potent ROS1 inhibitors.
BRAF: BRAF point mutation are found in 1-2% of pulmonary adenocarcinomas in both smokers and non-smokers. In about two-thirds of cases, a typical, treatable V600E mutation is present. Here, a combination treatment with dabrafenib and trametinib is the therapy of choice, both in therapy-naive and pretreated patients [55,56]. However, NSCLC with BRAF V600E also respond to chemotherapy and immunotherapy. For BRAF mutations other than V600E, targeted therapy outside of trials is not currently recommended.
KRAS: If an activating mutation of the Kirsten ras proto-oncogene (KRAS) is present, these NSCLC were previously not considered targetable and were referred to chemo-immunotherapy. KRAS was tested anyway because a positive result made the search for further driver mutations unnecessary. In 2019, initial results from a clinical trial with the specific KRAS G12C inhibitor AMG510 were reported [57]. These results are very encouraging and follow-up studies are now being opened at many centers for recruitment of a large number of patients. Other KRAS inhibitors are also in development.
RET: “Rearranged during transfection” (RET) translocations are found in approximately 1-2% of pulmonary adenocarcinomas. Less selective TKIs such as cabozantinib did show some effect, albeit very limited in time [58]. Highly selective RET inhibitors such as LOXO-292 and BLU-667 therefore represent a major advance in the treatment of RET-mutated lung tumors. LOXO-292 is being studied in the LIBRETTO-001 trial (NCT03157128). Early results showed a response rate of 65%, with very good tolerability [59]. New data will be presented at the IASLC Annual Congress in September 2019. Participating centers include Lucerne (CH) and Cologne (DE). The RET inhibitor BLU-667 is being studied in the ARROW trial (NCT03037385). The preliminal response rate was 56% [60]. Participating centers include Heidelberg (DE).
NTRK: Fusions of one of the proto-oncogenes “neurotrophic receptor thyrosine kinase 1-3” (NTRK 1-3) are found in 1% of NSCLC. The TRK inhibitor larotrectinib (LOXO-101) has shown an ORR of 75% in phase I-II studies to date, with sustained response and very good tolerability [61]. So far approved in the USA, approval for Europe and Switzerland is still pending. Entrectinib (RXDX-101), another TRK inhibitor showed an ORR of 57% in solid tumors with proven NTRK fusion in the combined analysis of three phase I-II studies with also very good tolerability [62].
Conclusion
Current developments in immunotherapy and targeted therapy show that the potential of these approaches in bronchial carcinoma is far from exhausted. The benefit of these therapies in palliation is undisputed and clinical trials are also ongoing in the adjuvant setting. Regardless of this, increased efforts must also be made in the areas of prevention and early detection so that a decrease in bronchial carcinomas (especially metastatic stages) can be achieved.
Take-Home Messages
- Bronchial carcinoma is the leading cause of cancer-related deaths worldwide, and tobacco smoking is the leading cause.
- The new standard of care for stage IV small cell lung cancer (SCLC) is the combination of chemotherapy (carboplatin and
- Etoposide) with the immune checkpoint inhibitor atezolizumab.
- Patients with stage III non-small cell lung cancer (NSCLC) benefit from consolidative immunotherapy with durvalumab after definitive radio/chemotherapy.
- Testing of advanced stage non-squamous NSCLC for the presence of driver mutations (in advance EGFR, ALK, ROS1 and BRAF) and the level of PD-L1 expression is seminal for the choice of therapy.
- NSCLC with treatable mutations should be targeted in the first instance; treatment of NSCLC without evidence of a treatable driver mutation is based primarily on PD-L1 expression status.
Literature:
- Bray F, et al: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 2018. 68(6): p. 394-424.
- www.bfs.admin.ch/bfs/de/home/statistiken/kataloge-datenbanken/tabellen.assetdetail.6466432.html.
- Arndt V: Swiss Cancer Report 2015: Status and Developments. 2016: Office féderal de la statistique (OFS).
- Forman D, et al: The global and regional burden of cancer. World cancer report, 2014. 2014: 16-53.
- Sun S, Schiller JH, Gazdar AF: Lung cancer in never smokers – a different disease. Nat Rev Cancer, 2007. 7(10): 778-790.
- van Meerbeeck JP, Fennell DA, De Ruysscher DK: Small-cell lung cancer. Lancet, 2011. 378(9804): 1741-1755.
- Yang CF, et al: Role of Adjuvant Therapy in a Population-Based Cohort of Patients With Early-Stage Small-Cell Lung Cancer. J Clin Oncol, 2016. 34(10): 1057-1064.
- Warde P, Payne D: Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol, 1992. 10(6): 890-895.
- Pignon JP, et al: A Meta-Analysis of Thoracic Radiotherapy for Small-Cell Lung Cancer. New England Journal of Medicine, 1992. 327(23): 1618-1624.
- Mascaux C., et al: A systematic review of the role of etoposide and cisplatin in the chemotherapy of small cell lung cancer with methodology assessment and meta-analysis. Lung Cancer, 2000. 30(1): 23-36.
- Rossi A, et al: Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J Clin Oncol, 2012. 30(14): 1692-1698.
- Slotman BJ, et al: Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet, 2015. 385(9962): 36-42.
- Horn L, et al: First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med, 2018. 379(23): 2220-2229.
- www.astrazeneca.com/media-centre/press-releases/2019/imfinzi-improves-overall-survival-at-interim-analysis-in-the-phase-iii-caspian-trial-in-1st-line-extensive-stage-small-cell-lung-cancer-27062019.html.
- Auperin A, et al: Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med, 1999. 341(7): 476-484.
- Slotman B, et al: Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med, 2007. 357(7): 664-672.
- Takahashi T, et al: Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol, 2017. 18(5): 663-671.
- Gjyshi O, et al: Evolving Practice Patterns in the Use of Prophylactic Cranial Irradiation for Extensive-Stage Small Cell Lung CancerProphylactic Cranial Irradiation for Extensive-Stage Small Cell Lung Cancer. JAMA Network Open, 2019. 2(8): e199135-e199135.
- Böttger F, et al: Tumor Heterogeneity Underlies Differential Cisplatin Sensitivity in Mouse Models of Small-Cell Lung Cancer. Cell reports, 2019. 27(11): 3345-3358. e4.
- O’Brien ME, et al: Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol, 2006. 24(34): 5441-5447.
- von Pawel J, et al: Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol, 1999. 17(2): 658-667.
- Eckardt JR, et al: Open-label, multicenter, randomized, phase III study comparing oral topotecan/cisplatin versus etoposide/cisplatin as treatment for chemotherapy-naive patients with extensive-disease small-cell lung cancer. J Clin Oncol, 2006. 24(13): 2044-2051.
- Garassino MC, et al: Outcomes of small-cell lung cancer patients treated with second-line chemotherapy: a multi-institutional retrospective analysis. Lung Cancer, 2011. 72(3): 378-83.
- Chung HC, et al: Abstract CT073: Pembrolizumab after two or more lines of prior therapy in patients with advanced small-cell lung cancer (SCLC): Results from the KEYNOTE-028 and KEYNOTE-158 studies. Cancer Research, 2019. 79(13 Suppl): CT073-CT073.
- Ready N, et al: Third-line nivolumab monotherapy in recurrent SCLC: CheckMate 032. J Thorac Oncol, 2019. 14(2): 237-244.
- Pless M., et al: Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. The Lancet, 2015. 386(9998): 1049-1056.
- Burdett S, et al: Postoperative radiotherapy for non-small cell lung cancer. Cochrane database of systematic reviews, 2016(10).
- Antonia SJ, et al: Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med, 2017. 377(20): 1919-1929.
- Antonia SJ, et al: Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med, 2018. 379(24): 2342-2350.
- Reck M, et al: Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med, 2016. 375(19): 1823-1833.
- Gandhi L, et al: Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med, 2018. 378(22): 2078-2092.
- Socinski, M.A., et al: Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med, 2018. 378(24): 2288-2301.
- West H., et al: Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol, 2019. 20(7): 924-937.
- Paz-Ares L, et al: Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med, 2018. 379(21): 2040-2051.
- Vokes EE, et al: Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol, 2018. 29(4): 959-965.
- Rittmeyer A, et al: Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet, 2017. 389(10066): 255-265.
- Fehrenbacher L, et al: Updated Efficacy Analysis Including Secondary Population Results for OAK: A Randomized Phase III Study of Atezolizumab versus Docetaxel in Patients with Previously Treated Advanced Non-Small Cell Lung Cancer. J Thorac Oncol, 2018. 13(8): 1156-1170.
- Herbst RS, et al: Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet, 2016. 387(10027): 1540-1550.
- Alexandrov LB, et al: Signatures of mutational processes in human cancer. Nature, 2013. 500(7463): 415.
- Shi Y, et al: A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol, 2014. 9(2): 154-162.
- Mitsudomi T, Yatabe Y: Epidermal growth factor receptor in relation to tumor development: EGFR gene and cancer. The FEBS journal, 2010. 277(2): 301-308.
- Lee CK, et al: Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst, 2013. 105(9): 595-605.
- Wu SG, Shih J-Y, Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Molecular cancer, 2018. 17(1): 38.
- Soria JC, et al: Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. New England journal of medicine, 2018. 378(2): 113-125.
- Tagrisso significantly improves overall survival in the phase III FLAURA trial for 1st-line EGFR-mutated non-small cell lung cancer [press release]. Wilmington, DE: AstraZeneca; August 9, 2019. https://bit.ly/2ZGF7gZ.
- Nakamura A, et al: Phase III study comparing gefitinib monotherapy (G) to combination therapy with gefitinib, carboplatin, and pemetrexed (GCP) for untreated patients (pts) with advanced non-small cell lung cancer (NSCLC) with EGFR mutations (NEJ009). Journal of Clinical Oncology, 2018. 36(15_suppl): 9005-9005.
- Saito H, et al: Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interimanalysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol, 2019. 20(5): 625-635.
- Soda M, et al: Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature, 2007. 448(7153): 561.
- Shaw AT, et al: Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. Journal of clinical oncology, 2009. 27(26): 4247.
- Peters S, et al: Alectinib versus crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med, 2017. 377(9): 829-838.
- Hida T, et al: Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet, 2017. 390(10089): 29-39.
- Ryser CO, Diebold J, Gautschi O: Treatment of anaplastic lymphoma kinase-positive non-small cell lung cancer: update and perspectives. Curr Opin Oncol, 2019. 31(1): 8-12.
- Shaw AT, et al: Crizotinib in ROS1-rearranged non-small-cell lung cancer. New England Journal of Medicine, 2014. 371(21): 1963-1971.
- Ou S, et al: OA02. 03 Clinical Activity of Lorlatinib in Patients with ROS1+ Advanced Non-Small Cell Lung Cancer: Phase 2 Study Cohort EXP-6. Journal of Thoracic Oncology, 2018. 13(10): S322-S323.
- Planchard D, et al: Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol, 2016. 17(7): 984-993.
- Planchard D, et al: Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol, 2017. 18(10): 1307-1316.
- Fakih M, et al: Phase 1 study evaluating the safety, tolerability, pharmacokinetics (PK), and efficacy of AMG 510, a novel small molecule KRASG12C inhibitor, in advanced solid tumors. Journal of Clinical Oncology, 2019. 37(15_suppl): 3003-3003.
- Gautschi O, et al: Targeting RET in Patients With RET-Rearranged Lung Cancers: Results From the Global, Multicenter RET Registry. J Clin Oncol, 2017. 35(13): 1403-1410.
- Drilon AE, et al: A phase 1 study of LOXO-292, a potent and highly selective RET inhibitor, in patients with RET-altered cancers. Journal of Clinical Oncology, 2018. 36(15_suppl): 102-102.
- Gainor JF, et al: Clinical activity and tolerability of BLU-667, a highly potent and selective RET inhibitor, in patients (pts) with advanced RET-fusion+ non-small cell lung cancer (NSCLC). Journal of Clinical Oncology, 2019. 37(15_suppl): 9008-9008.
- Drilon A, et al: Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. New England Journal of Medicine, 2018. 378(8): 731-739.
- Demetri GD, et al: LBA17Efficacy and safety of entrectinib in patients with NTRK fusion-positive (NTRK-fp) tumors: pooled analysis of STARTRK-2, STARTRK-1 and ALKA-372-001. Annals of Oncology, 2018. 29(suppl_8).
- “DeVita,” Hellman and Rosenberg’s Cancer: Principles & Practice of Oncology, 11th edition; Wolters Kluwer, 2019.
InFo ONCOLOGY & HEMATOLOGY 2019; 7(4): 12-17.