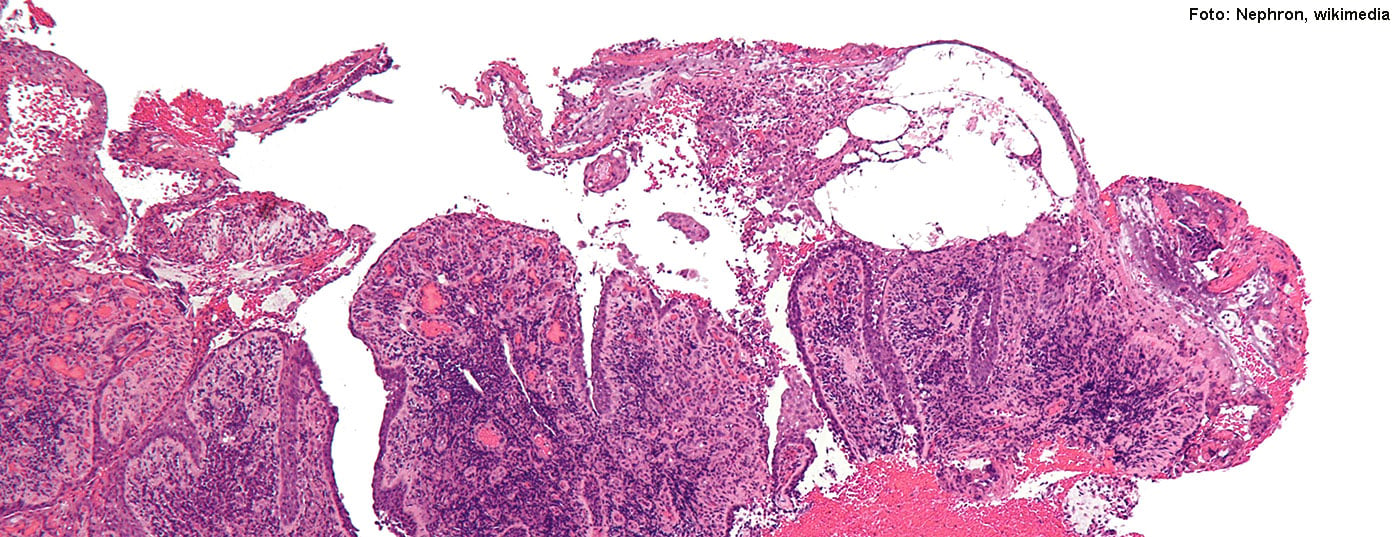
In recent years, great progress has been made in the study of autologous immunocellular therapies. The development of a target-specific chimeric antigen receptor (CAR) against pathogenetic autoreactive B-cell pemphigus vulgaris is one of these milestones.
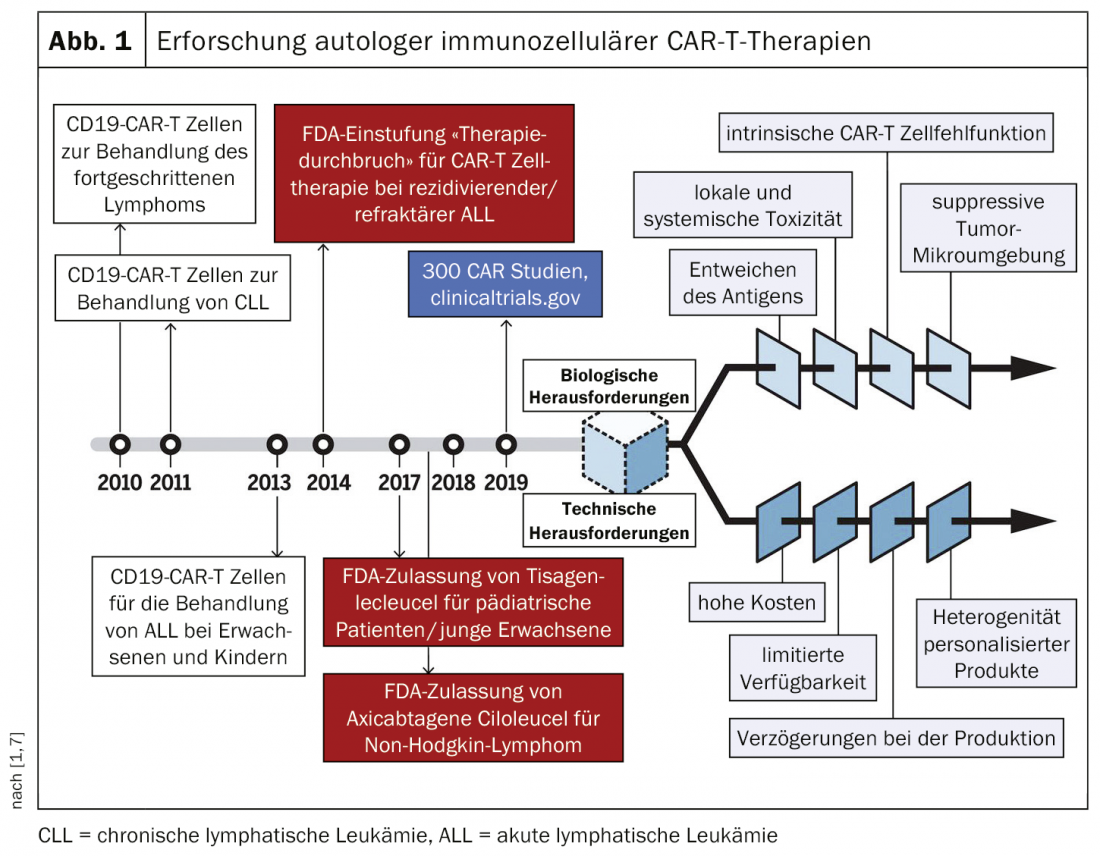
Immunotherapies are a technique in which T lymphocytes are genetically modified so that they selectively destroy pathogenetic cellular material. Christoph Ellebrecht, MD, University of Pennsylvania (USA) gave a presentation at this year’s annual meeting of the Arbeitsgemeinschaft Dermatologische Forschung (ADF) [1] on the target-specific immunotherapies CAR-T (“Chimeric antigen receptor T-cell therapy”) and CAAR-T (“Chimeric autoantibody receptor T-cell therapy”) in the rare, blistering, and potentially fatal autoimmune disease pemphigus vulgaris (PV) [2–4]. Several studies have demonstrated that chimeric antigen receptor cells are effective as target-specific therapy (CAR-T) against pathogenic B cells in PV. The CAR-T cell concept has been explored for several decades, mainly in the field of anti-cancer therapies, with technical challenges delaying market breakthrough (Fig. 1) . In 2018, a CAR-T product received marketing approval in Switzerland in the oncology field (tisagenlecleucel: for refractory cases of leukemia and large B-cell lymphoma) [5].
Anti-CD 20 therapy is usually only effective in the short term
If left untreated, PV is fatal after a few years as a result of fluid loss and superinfection through the damaged skin barrier. Treatment options used to date include systemic corticosteroids, azathioprine, mycophenolate mofetil, cyclophosphamide, methotrexate. In March 2019, the monoclonal antibody rituximab received marketing authorization in the EU for moderate to severe forms of PV; in the US, this indication extension was already granted in 2018. Rituximab is a biologic that specifically targets CD20 by binding to this transmembrane antigen localized on pre-B and mature B lymphocytes. The speaker explained that rituximab is a short-term therapeutic strategy, but that the recurrence rate is relatively high. Thus, although disease activity can be controlled by destroying memory B cells in 95% of cases, relapse occurs in 77% [6]. It is known that autoreactive B cells play a key role in the pathomechanism of recurrences.
CAR-T and CAAR-T in pemphigus vulgaris
To treat PV without causing widespread immunosuppression, the University of Pennsylvania research team developed an artificial chimeric autoantibody receptor (CAAR) that displays fragments of the autoantigen Dsg3 – the same fragments that PV-causing antibodies and their B cells typically bind to. Autoreactive B cells play an important role in the pathomechanism of PV and their specific elimination is a central mechanism of action of this treatment strategy. A receptor design proven in cell culture was also successful in a mouse model for PV. In vivo studies showed that CAAR T cells recognize and specifically eliminate Dsg3-reactive B cells and do not lead to off-target toxicity against keratinocytes [1].
In contrast to antibody-based therapy, in which a defined dose is infused, CAAR T cells can expand several-fold in vivo and give rise to T cells that persist for decades. Thus, CAAR T cells need to be infused only once, they kill all pemphigus B cells and then become inactive. If a pemphigus B cell recurs at any time in the future, the memory CAAR T cells may again enlarge and eliminate it.
A review article published in 2018 by Siddiqi et al. [4] agrees that CAR-T is a promising treatment approach for PV, but the authors point out that current CAR-T therapies are associated with a relatively high risk of cytokine release syndrome as an adverse side effect, a potentially fatal systemic inflammatory disease.
The development and market approval of CAR-T products in the oncology field has also faced hurdles, but some compounds are now available. Tisagenlecleucel, a CAR-T product approved in Switzerland for the treatment of refractory B-cell acute lymphoblastic leukemia (adolescents) and refractory large B-cell lymphoma (adults) since 2018, is an autologous immunocellular cancer therapy that results in reprogramming of patient-derived T cells with a transgene encoding a chimeric antigen receptor (CAR) so that they identify and eliminate CD19-expressing cells [5].
Literature:
- Ellebrecht CT: Go CAART – Immunotherapy for non-cancer applications, Christoph Ellebrecht, MD, Dermatology resident, University of Pennsylvania, slide presentation 46th Annual Meeting of the Association for Dermatologic Research (ADF), March 13-16, 2019, Munich, Germany.
- Ellebrecht CT, Payne AS: Setting the target for pemphigus vulgaris therapy. JCI Insight 2017; 2(5): e92021. https://doi.org/10.1172/jci.insight.92021.
- Ellebrecht CT, et al: Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science 2016; 353(6295): 179-184. doi: 10.1126/science.aaf6756. Epub 2016 Jun 30.
- Siddiqi HF, Staser KW, Nambudiri VE: Research Techniques Made Simple: CAR T-Cell Therapy. Journal of Investigative Dermatology 2018; 138: 2501e2504. doi:10.1016/j.jid.2018.09.002. https://beaumont.cloud-cme.com/assets/beaumont/Presentations/14208/14208.pdf
- Compendium: KYMRIAH, https://compendium.ch/prod/kymriah-zellsuspension-inf-los–nh-/de, last accessed July 15, 2019.
- Joly P: First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randofmised trial. Lancet 2017; 389(10083): 2031-2040. doi: 10.1016/S0140-6736(17)30070-3. Epub 2017 Mar 22.
- Schultz L, et al: Driving CAR T cell translation forward. Science Translational Medicine 2019; 11 (481), eaaw2127. DOI: 10.1126/scitranslmed.aaw2127
DERMATOLOGIE PRAXIS 2019; 29(4): 46-47 (published 8/28/19, ahead of print).