Medical advances have also had an impact on the epidemiology of infective endocarditis. Especially catheter valve implantations (TAVI) will be a major challenge in the future.
Infective endocarditis refers to an infection of the endocardium (especially the heart valves) or of intracardiac implants (for example, artificial heart valves, aortic prostheses, and electrodes of pacemakers and defibrillators) caused by bacteria in more than 90% of cases. In clinical practice, the aortic and mitral valves are most commonly affected. The right-sided heart valves are involved in only about 5-10% of all cases, here mostly the tricuspid valve. Risk factors for right-sided endocarditis are primarily intravenous drug abuse, as well as intracardiac devices such as pacing leads and intravascular catheters (especially central venous catheters). Isolated congenital heart defects (especially those with valvular pathology and/or with a shunt) also pose a risk for the occurrence of endocarditis [1,2]. Prosthetic valve endocarditis accounts for approximately 5% of all infective endocarditis [1–4].
Prophylaxis
Infective endocarditis was first described by William Osler [5] in 1885. In the 1920s, bacteria circulating in the bloodstream after dental procedures were first suspected as a cause of valve infection, and accordingly, the first recommendations for antibiotic prophylaxis were published by the AHA (American Heart Association) in 1955 [6]. Since then, recommendations for prophylactic antibiotic administration have been adjusted several times. This primarily involves weighing the benefits of prophylaxis against the risk of promoting antibiotic resistance. In the last revision in 2007, the recommendations for antibiotic prophylaxis in risk situations were very restrictive (Tab. 1) [7,8].

In particular, the number and type of procedures and actions for which prophylaxis should be administered were restricted. Antibiotic prophylaxis is still indicated for dental procedures involving manipulation of the gingival sulcus or perforation of the mucosa, as well as for procedures in the oropharyngeal region if the mucosa is perforated. In contrast, the recommendation for prophylaxis during procedures and manipulations of the urogenital tract, gynecologic procedures, and gastrointestinal procedures such as endoscopies has been withdrawn, provided there is no active infection in these organ systems. Otherwise, prophylaxis and appropriate therapy are of course necessary.
The risk groups of patients who should continue to receive prophylaxis were also defined much more narrowly. At present, only patients at highest risk should receive prophylaxis and, in particular, those patients at high risk of an unfavorable course if infective endocarditis occurs. This includes all patients after valve replacement (whether surgical with mechanical or biological prosthesis or interventional with transcatheter valves). Risk groups also include patients who have already undergone endocarditis, patients with congenital vitiation (see separate lists in the guidelines [7,8]), and patients after heart transplantation with new-onset valvulopathy. In patients with condition after valve reconstruction, prophylaxis is recommended for 6 months until endothelialization. In contrast, antibiotic prophylaxis is no longer indicated in patients with a valvular defect, such as aortic stenosis or mitral regurgitation.
The risk of increasing antibiotic resistance in the most common types of germs was cited as the rationale for a much more restrictive approach to prophylactic antibiotic administration. Indeed, there are reports showing a decrease in resistant germs to about 10% since the introduction of the new guidelines, although causality is difficult to prove [10]. The economic factor and patient safety should also not be underestimated, since serious allergic reactions may well occur even during antibiotic prohylaxis. As a further justification for the more restrictive stance, studies are highlighted that show bacteremia during dental hygiene even during normal tooth brushing, which is similar in magnitude to that seen in dental procedures without involvement of the gingival apparatus. Therefore, prophylaxis is not warranted in situations that do not exceed the risk level of normal everyday risk.
Incidence
The incidence of infective endocarditis is 3-10 per 100,000 persons per year. Nearly 40% of prosthetic valve infections are now hospital-associated. Hospital-associated means that an infection arises in connection with a hospital stay (for example, from venous catheters inserted, wound infections, pneumonia, urinary tract infections). In addition, there are numerous publications reporting an increase in incidence in recent years [1,2,9,10]. We can very well confirm this observation based on our own experience. However, interpreting the increase in cases with prosthetic endocarditis is difficult because many factors can influence these numbers. In a study from England, after restriction of endocarditis prophylaxis, the number of infections and antibiotic prescriptions were analyzed using a national registry. A dramatic decrease in antibiotic prescriptions was shown but at the same time a rapid increase in endocarditis cases [9].
Other studies in other countries have not demonstrated a similarly rapid increase in endocarditis cases, which is why the authors of the English study themselves qualify the reference to the changed prophylaxis guidelines [6].
The increasing age of patients, combined with correspondingly more and more complex comorbidities is likely to be one of the most important changes explaining the significant increase in cases of infective endocarditis. In the past, younger patients with rheumatic valve disease were predisposed to endocarditis, whereas nowadays, in regions with good medical care, mainly older patients with degenerative valve disease are affected. However, due to migration, a renewed increase in the prevalence of rheumatic valve disease with corresponding predisposition is observed in our hospitals. Other important risk factors include the increasing use of procedures, such as dialysis and immunosuppression, and the increase in patients who have had intravascular catheters (central venous catheters), intracardiac implants such as pacemakers, other foreign materials such as hip and knee prostheses, and, of course, prosthetic valves installed [1,2,6,11,12]. In younger patients, it is mainly people with intravenous drug administration.
Diagnosis
Clinical findings, microbiological results, and imaging results must be combined when making a diagnosis. Here, Dukes criteria should be used (Table 2) [1,2,7,13], which can interpret different modalities and estimate the probability of endocarditis. An increasingly important role is attributed to imaging, with transesophageal echocardiography becoming the gold standard; with technological advances and increasing image resolution in terms of quality, an increased detection rate may be expected, and with it an increase in the number of cases. PET-CT diagnostics are increasingly used in cases of suspected infection of vascular prostheses, although much experience still needs to be gained in the evaluation of these examination findings.
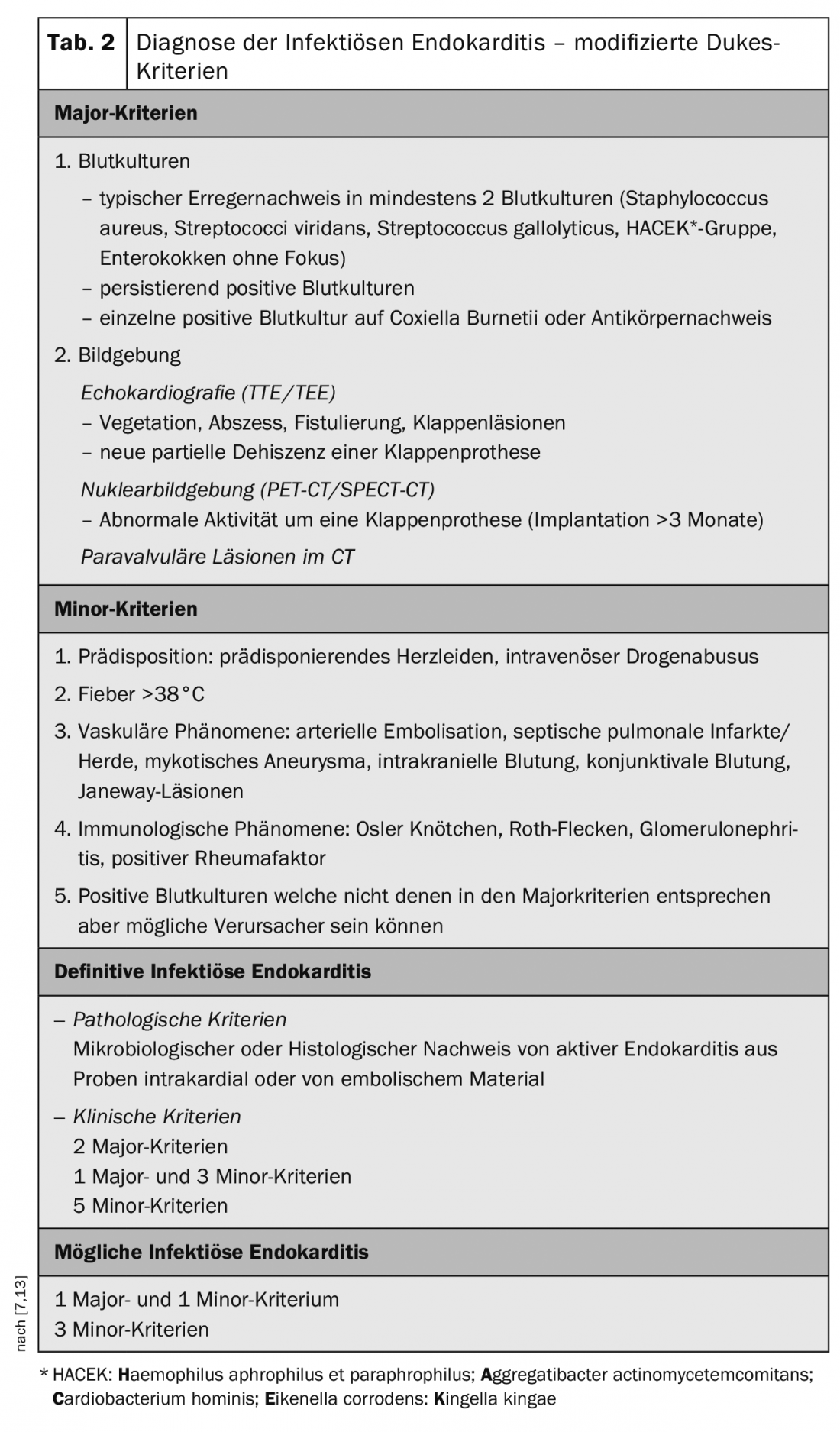
In the microbiological examinations it is important to take 3 sets of blood cultures whereby these must be taken quickly at the slightest suspicion. This triple cultivation captures up to 98% of the germs in bacteremia [7,14].
Microbiology
The spectrum of pathogens has changed in recent years from the previously most common pathogens from the oropharyngeal region to hospital-associated pathogens. Staphylococcus aureus has overtaken streptococci as the most common pathogen. In the 1960s, the proportion of Staphylococcus aureus was 18%, but this has since risen to as much as 38% (Table 3) [1,2,4,15].
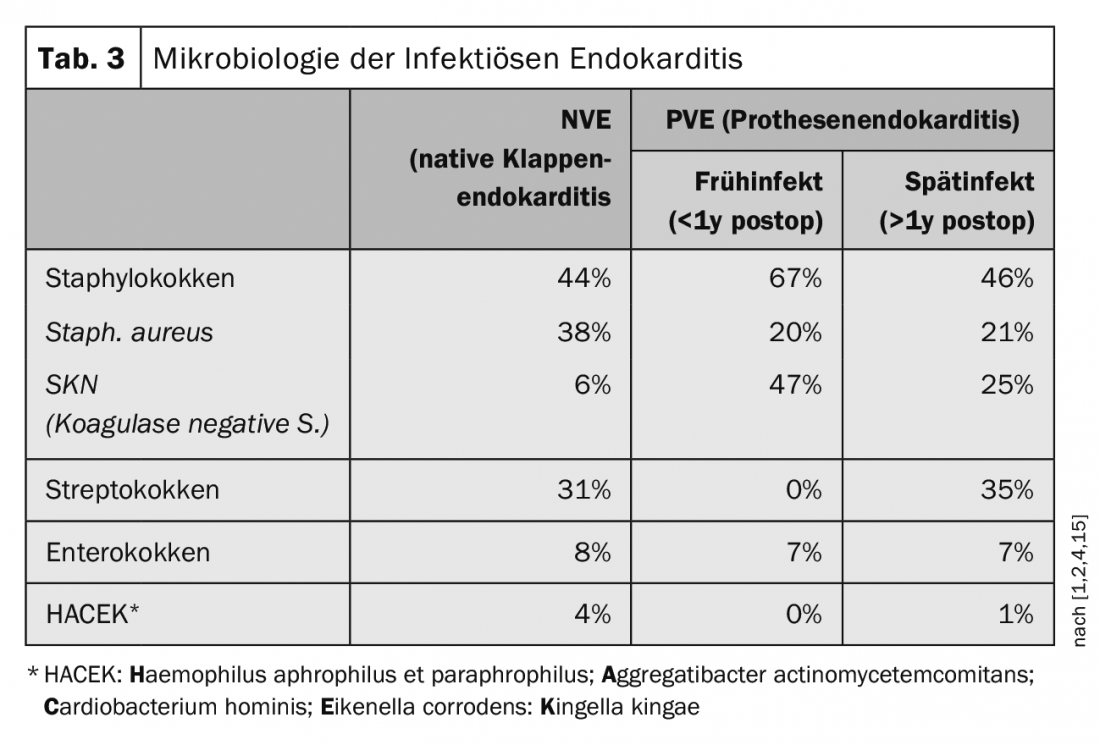
Infectious endocarditis of prosthetic valves, the so-called prosthetic valve endocarditis (PVE), is distinguished from the early infection within one year after implantation and the late infection, which occurs after one year after implantation [7,14]. Early infection occurs in up to 16% of prosthetic infections. Early infections directly related to surgery usually occur within 2-3 months and are most commonly caused by a coagulase negative staphylococcus (SKN) or a staphylococcus aureus. In transfemoral catheter valve implantation (TAVI), on the other hand, enterococci are more frequently isolated in early infections, although the data here are still relatively sparse [1,2]. Prosthetic valve infections occurring after one year are hematogenously caused infections and correspond to the germ spectrum of native valve endocarditis [16]. After implantation of a transfemoral valve prosthesis (TAVI), there is a 5-6% risk of endocarditis within 5 years. In comparison, there is a 2% risk of developing endocarditis within 5 years after implantation of a pacemaker. For surgical valve prostheses, whether biological or mechanical, the risk is about 3-4% within 5 years.
Despite all the advances in cardiac surgery, infectious diseases, and intensive care medicine, mortality has not changed significantly in recent decades: infective endocarditis remains a severe systemic disease with correspondingly high morbidity and mortality. Mortality in infective endocarditis is 20% and increases to over 50% in prosthetic endocarditis.
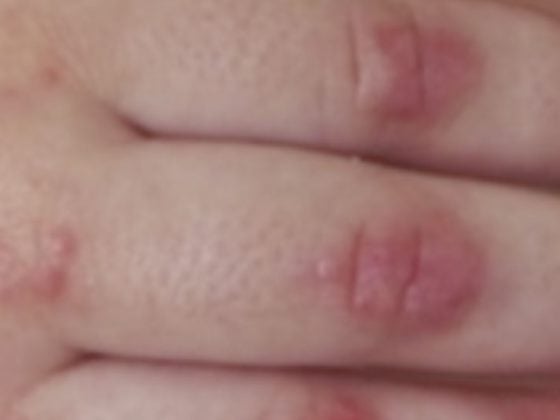
When a native heart valve is affected, there are usually primarily deposits, so-called vegetations, which, depending on their size, can embolize and, in the worst case, lead to a septic cerebrovascular insult. Furthermore, acute insufficiency of the affected valve – by destruction of the structure – may occur, leading to acute hemodynamic deterioration. Stenosis is observed much less frequently, occasionally in association with large vegetations.
In prosthetic endocarditis, vegetations can cause both stenosis and insufficiency in mechanical valves by blocking a valve leaflet. In biological valves, destruction of the valve pockets often occurs, leading to severe insufficiencies. A feared complication of prosthetic endocarditis is paravalvular insufficiency due to abscessation around the valve annulus. This can lead to complete loosening of the valve with the corresponding “rocking valve” phenomenon. Abscession is most commonly observed in endocarditis with Staphylococcus aureus.
Left-sided valve abscessation not infrequently occurs in the aortomitral junction, with corresponding destruction of ventriculo-aortic continuity and in the region of the membranous septum with corresponding disruption of AV conduction, up to and including third-degree AV block.
Surgery
Surgical therapy for endocarditis of native valves is necessary in 40-50% of cases; conservative therapy with antibiosis may result in healing of the affected valve in up to 60%. In the case of prosthetic valves, the situation is much more dangerous and surgical repair is required much more frequently. Pacemaker systems must also be upgraded in such patients. Reimplantation of a new pacemaker system depends on the particular and current indication and can be performed in the same operation using epicardial leads. The extravascular course of the latter makes them less susceptible to reinfection.
For patients requiring intensive care, transfer to the center with appropriate interdisciplinary treatment and assessment is important. In addition, studies show that an indication as soon as possible with appropriate performance of the operation brings a significantly better survival perioperatively, but also in the long-term course [17,18].
For the indication of surgical therapy, the European guidelines are guiding (Table 4) [7]. Surgical therapy is particularly indicated in the following situations: [2,3,7]
- Valve dysfunction with hemodynamic instability,
- uncontrollable infection despite adequate antibiotic therapy,
- Increasing abscessation with paravalvular insufficiency,
- Embolization prevention
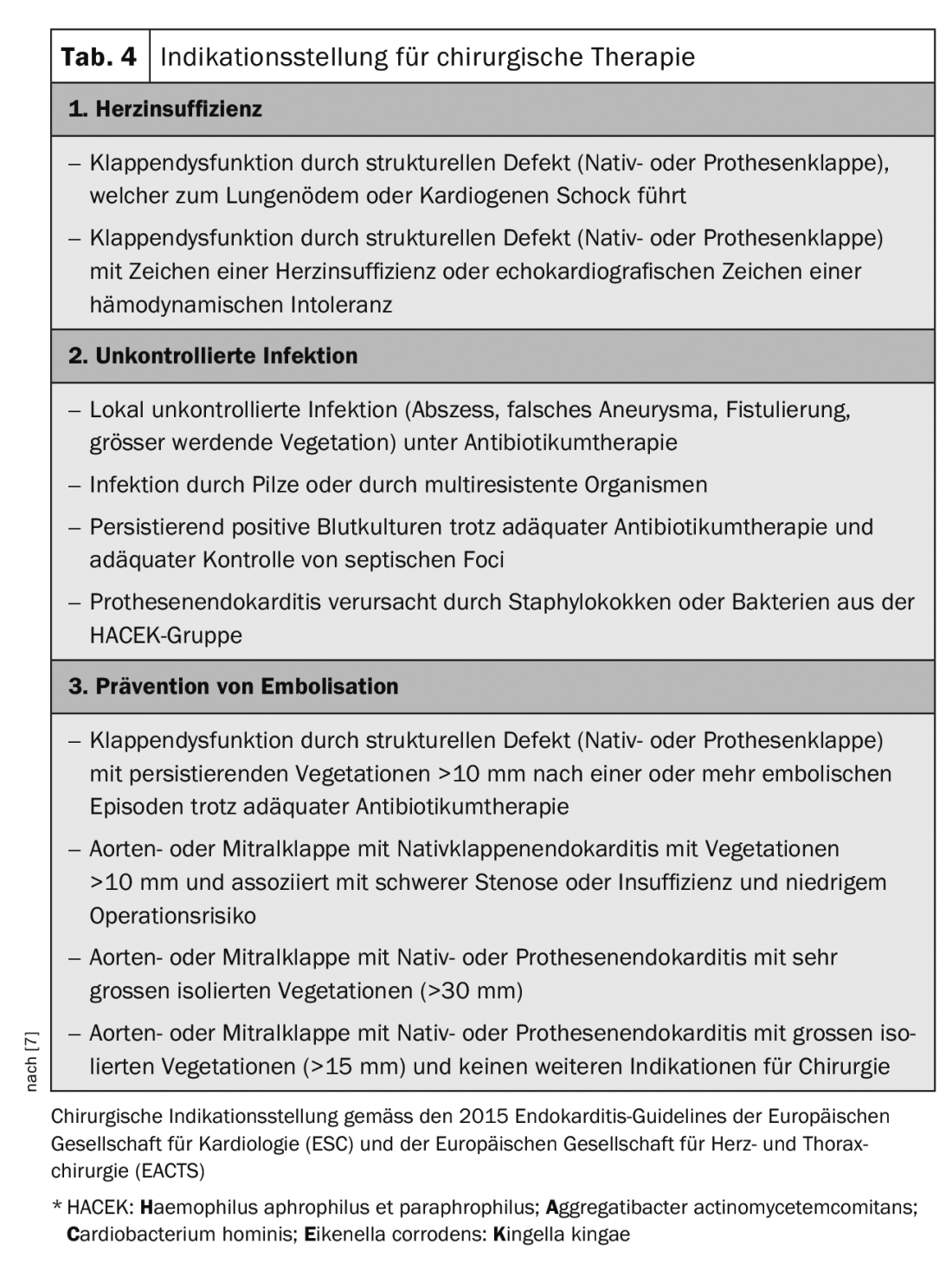
Regarding the prevention of embolization, there is room for improvement in the guidelines as well. Left-sided vegetations larger than 10 mm should be more generously indicated for early surgery [17]. In patients with stroke, surgery is often delayed too long, thus accepting further deterioration of the patient with further risk of embolism. After stroke without intracranial hemorrhage and without severe neurological impairment, surgery should be performed without delay. In case of large cerebral ischemia or hemorrhage, it must be considered whether to wait for at least 3-4 weeks.
In endocarditis of the native aortic valve, valve replacement is the treatment of choice. If the native mitral valve is affected, reconstruction can be attempted, provided the tissue is not completely macerated and/or destroyed. Infected prosthetic valves must be replaced in any case. Abscess cavities are usually closed with pericardial patches.
In the future, endocarditis of transcatheter valves is likely to become an increasing problem. Until recently, these valves were implanted primarily in elderly, inoperable patients who often died before developing endocarditis. And if endocariditis occurred, these patients were treated conservatively. With an expected number of younger patients receiving catheter valves in the future, the number of prosthetic endocarditis after TAVI will also increase. Indeed, it is likely that the incidence will be at least equal to that of conventional surgical prostheses.
Technically, interventions after implantation of a TAVI prosthesis may be challenging because these valves contain much more foreign material and, in particular, wire mesh, which often extends into or even beyond the aortic root. These can occasionally be extremely difficult to remove. After surgical valve replacement, on the other hand, the valve ring can usually be detached and a new valve implanted in the same place. With catheter valves, the risk of damage to the aortic root is greater. Also, if a composite graft eventually has to be implanted, the expense is significantly greater. The recurrence rate after prosthetic valve endocarditis is 6-15% [2].
The most important lesson in patients with an unexplained febrile state and a potential constellation of risk (prosthetic valve wearers, valvular vitium) is to think of the possibility of infective endocarditis, even if the symptoms are not quite typical. Especially in patients with prosthetic valves, endocarditis must be ruled out in any case of unclear fever. If there are signs of infection or decreased general health with a new heart murmur, it is also essential to look for endocarditis. In the future, the number of patients at risk (those with TAVI, vascular prostheses of the large arteries, or with endovascular stent grafts in the aorta) is likely to increase significantly.
It is also important to care for patients with cardiovascular implants, in terms of instruction regarding endocarditis prophylaxis – not only by means of antibiotics when indicated, but also in terms of hygienic measures such as dental care, but also the prevention and treatment of recurrent skin lesions or chronic wounds.
Take-Home Messages
- Medical advances are changing the epidemiology of infective endocarditis.
- Overall, we anticipate an increase in endocarditis cases. Diagnosis remains a challenge.
- Hospital-associated infections have also become an important problem in infective endocarditis.
- Surgical therapy of infective endocarditis becomes increasingly complex the more foreign material is already in the patient.
- Endocarditis after catheter valve implantation (TAVI) will be a major challenge in the future.
- Indication for administration of antibiotics must always be well justified.
- Therefore, the administration of endocarditis prophylaxis is also only recommended very restrictively.
Literature:
- Cahill TJ, et al: Challenges in infective endocarditis. JACC 2017; 69: 325-344.
- Cahill TJ, Prendergast BD: Infective endocarditis. Lancet 2016; 387: 882-893.
- Wang A, et al: Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA 2007; 297: 1354-1361.
- Moreillon P, Que YA: Infective endocarditis. Lancet 2004; 363: 139-149.
- Osler W.: The Gulstonian Lectures, on Malignant Endocarditis. BMJ 1985; 1: 577-579.
- Dayer M, Thornhill M: Antibiotic Prophylaxis Guidelines and Infective Endocarditis: Cause for Concern? JACC 2015; 65: 2077-2078.
- Habib G, et al: 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36: 3075-3128.
- Flückiger U, Jaussi A: Revised Swiss guidelines for endocarditis prophylaxis. Cardiovascular Medicine 2008; 11: 392-400.
- Dayer MJ, et al: Incidence of infective endocarditis in England, 2000-13: a secular trend, interrupted time-series analysis. Lancet 2015; 385: 1219-1228.
- Ostovar R, et al: Endocarditis: An Ever Increasing Problem in Cardiac Surgery. Thorac Cardiovasc Surg 2019; Epub ahead of print
- Slipczuk L, et al: Infective endocarditis epidemiology over five decades: a systematic review. PLoS One 2013; 8:e82665.
- Fefer P, et al: Changing epidemiology of infective endocarditis: a retrospective survey of 108 cases, 1990-1999. Eur J Microbiol Infect Dis 2002; 21: 432-437.
- Li JS, et al: Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30: 633-638.
- Vongpatanasin W, et al: Prosthetic heart valves. NEJM 1996; 335: 407-416.
- Murdoch DR, et al: Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis – Prospective Cohort Study. Arch Intern Med 2009; 169: 463-473.
- Butt JH, et al: Long-Term Risk of Infective Endocarditis After Transcatheter Aortic Valve Replacement. JACC 2019; 73: 1646-1655.
- Carrel T.: Early valve repair or replacement is not generally contraindicated in patients with infective endocarditis and stroke with or without intracranial haemorrhage. Eur J Cardiothorac Surg 2016; 50: 383-384.
- Carrel T, et al: What’s new in surgical treatment of infective endocarditis? Intensive Care Med 2016; 42: 2052-2054.
CARDIOVASC 2019; 18(4): 9-13