In recent years, late-onset MS and age-correlated effects on disease progression have become an increasing focus of interest. There are many new findings, but also some unanswered questions.
Melinda Magyari, MD, PhD, assistant professor at The Danish Multiple Sclerosis Center, presented on late-onset MS and findings on age-correlated characteristics of this chronic central nervous system disease. Epidemiological studies show that the prevalence and life expectancy of MS have increased worldwide [1] (box) . The demographic structure of the patient population has changed, which is reflected, among other things, in a higher mean age of those affected.
Disease progression correlates with age of initial manifestation
Higher age at disease onset is associated with a shorter interval to disease progression, the speaker explained. A strong independent correlation of age and disease progression could be demonstrated empirically [5]. Accelerated age-related mechanisms in the central nervous system are thought to play a role in this pattern, in addition to acute focal recurrent inflammation and diffuse chronic neurodegeneration [5]. According to a 2017 review, age-related change in the immune system (“immunosenescence”) is a factor relevant to neuroinflammatory processes. Changes in the adaptive and innate immune system during the aging process are a possible explanation for an increased risk of infections and infection-associated mortality in patients who develop MS at an older age. The authors summarize that when immunomodulatory and immunoprogressive medications are used, these additive or synergistic effects should be considered to avoid infection risks [6].
In a cross-sectional study published in 2016 by Alroughani et al. [7], the risk of achieving an Expanded Disability Status Scale (EDSS) score of 6.0 was examined among patients with late-onset MS (<40 years; n=99 ≙ 10.7%), and young-onset MS (18-40 years; n=804 ≙ 89.3%). Multivariate models (Cox proportional hazards) showed that patients with late-onset MS achieved an EDSS score of 6.0 within significantly shorter time, with older age at first onset of MS (*aHR≙R=3.96; 95% CI: 2.14-7.32; p<0.001), male sex (aHR=1.85; 95% CI: 1.22-2.81; p=0.004), and spinal cord condition at initial manifestation (aHR=1.47; 95% CI: 0.98-2.21; p= 0.062) were significantly correlated with a shorter time to EDSS 6.0.
* = aHR adjusted hazard ratio
Limited evidence base on the efficacy of DMT in older sufferers.
That the effects of progression-modifying pharmacologic therapy (DMT) are age-dependent has been shown in other secondary analyses, Dr. Magyari said. Starting therapy as early as possible is crucial, he said. In a meta-analysis on the age-correlated effects of MS medication, the results of a linear regression model indicate that there is no predictive benefit for immunomodulatory DMT in MS sufferers older than 53 years [8]. Data from an observational study published in 2019 suggest that DMT may reduce the risk of conversion from relapsing-remitting MS (RRMS) to secondary progressive (SPMS), and this effect was more pronounced when DMT therapy was initiated within five years of disease onset than later [9].
A substantial proportion of older people with MS are considering discontinuing DMT, and there is insufficient data to make evidence-based recommendations, she said. RCTs on the effect of DMT have often excluded older people with MS, and therefore there is a low overall evidence base for this patient population. Data on the safety and efficacy of current DMT regimens for people affected by late-onset MS are also lacking and studies specific to this population are needed. This is also consistent with the conclusion of a systematic review on this topic that there is a need for high-quality evidence regarding standardized incidence and prevalence rates of comorbidities in MS at all stages of the disease to inform associations and related implications [13].
Brain atrophy is a repeatedly demonstrated indicator of disease progression [10] and there is increasing evidence that DMT may have a beneficial effect on MS-related brain atrophy [10,11]. The speaker explained that SPMS patients have up to 10- and 14-fold higher gray matter atrophy compared to patients with RRMS and healthy controls [1]. An analysis of MRI data shows that brain atrophy occurs in all stages of MS and progresses gradually along with inflammatory lesions [11]. In healthy individuals, the rate of brain atrophy after age 35 is about 0.2% per year with a gradual increase to an annual rate of atrophy of 0.5% at age 60 [12].
Influence of comorbidities on mortality risk.
Life expectancy for people with MS has increased in the era of DMT, but remains reduced by several years. MS is known to be associated with an increased risk of comorbidities. A study from Finland demonstrated that decreased life expectancy (MS patients 82.4 years; healthy controls 85.6 years) was associated with increased risk of stroke (OR=1.49; 95% CI 1.03-2.35) and type 1 diabetes (OR=2.1; 1.3-3.36) [14].
Also, a study of people with MS in Denmark showed that chronic comorbidities are associated with an increased risk of mortality (hazard ratio) [15]: psychiatri-
disease (2.42 [1,67–3,01]; p<0.0005), cerebrovascular disease (2.47 [2,05–2,79]; p<0.0005), cardiovascular disease (1.68 [1,39–2,03]; p<0.0005), lung disease (1.23 [1,01–1,50]; p<0.05), diabetes (1.39 [1,05–1,85]; p<0.05), cancer (3.51 [2,94–4,19]; p<0.0005), Parkinson’s disease (2.85 [1,34–6,06]; p<0,01).
Danish Multiple Sclerosis Registry
In the Danish MS registry, almost all data of people affected since 1950 are recorded and prevalence figures as well as incidence rates standardized to the European standard population have been calculated [16,17]. This shows, among other things, that both prevalence and incidence (Fig. 1) and the average age at first manifestation have increased.
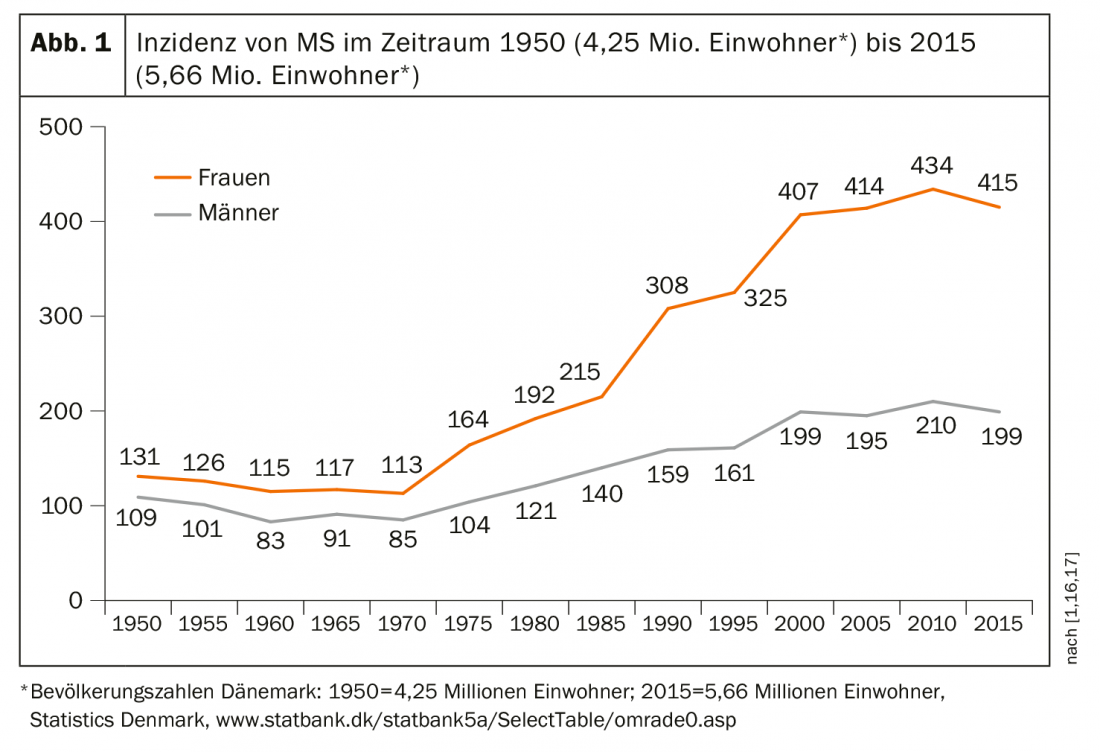
The relative incidence over the period from 1950 to 2009 (n=19,536) increased more than twofold in females from 5.91/100 000 (95% CI: 5.60-6.24) to 12.33/100 000 (95% CI: 11.91-12.75) per year. In males, there was a 24% increase from 4.52/100 000 (95% CI: 4.24-4.81) to 6.08 (95% CI: 5.79-6.38). When the age at first manifestation was 50 years and older, the incidence increased by a factor of 4.30 in women and by a factor of 2.72 in men. The authors suggest that among females, lifestyle changes including lower birth rates and increases in obesity and cigarette use may play a role.
Source: MS “State of the Art Symposium” 2019, Lucerne
Literature:
- Magyari M: Challenges in MS Treatment and Research, Melinda Magyari, MD, PhD, Ass. Prof, The Danish Multiple Sclerosis Center, “State of the Art Symposium,” Lucerne, Switzerland. Jan. 6, 2019-Jan. 26, 2019. www.multiplesklerose.ch/de/ueber-ms/fachkongresse/state-of-the-art/, last accessed 04 Jun. 2019.
- Rotstein DL, et al: Temporal trends in multiple sclerosis prevalence and incidence in a large population. Neurology 2018; 90 (16) :e1435-e1441. doi: 10.1212/WNL.00000000005331. PubMed PMID:29549225.
- Solaro C, et al: The changing face of multiple sclerosis: prevalence and incidence in an aging population. Mult Scler 2015; 21(10): 1244-50. doi: 10.1177/1352458514561904. Epub 2015 Jan 12. https://doi.org/10.1177/1352458514561904
- Warren SA, et al: Multiple sclerosis mortality rates in Canada, 1975-2009. Can J Neurol Sci 2016; 43(1):134-141. doi: 10.1017/cjn.2015.236. epub 2015 Aug 14.
- Confavreux C, Vukusic S: Age at disability milestones in multiple sclerosis. Brain 2006; 129(3): 595¬-605. Epub 2006 Jan 16.
- Grebenciucova E, Berger JR: Immunosenescence: the Role of Aging in the Predisposition to Neuro-Infectious Complications Arising from the Treatment of Multiple Sclerosis. Curr Neurol Neurosci Rep 2017; 17(8): 61.
- Alroughani R, et al: Is Time to Reach EDSS 6.0 Faster in Patients with Late-Onset versus Young-Onset Multiple Sclerosis? PLOS ONE 2016; https://doi.org/10.1371/journal.pone.0165846
- Weideman AM, et al: Meta-analysis of the Age-Dependent Efficacy of Multiple Sclerosis Treatments. Front Neurol 2017; 8: 577.
- Brown JWL, et al: Association of Initial Disease-Modifying Therapy With Later Conversion to Secondary Progressive Multiple Sclerosis. JAMA 2019; 321(2): 175.
- Zivadinov R, et al: Clinical relevance of brain atrophy assessment in multiple sclerosis. Implications for its use in a clinical routine. Expert Rev Neurother 2016; 16(7): 777-793.
- Miller DH, et al: Measurement of atrophy in multiple sclerosis: pathological basis, methodological aspects and clinical relevance. Brain 2002; 125: 1676-1695.
- Hedman AM, et al: Human brain changes across the life span: a review of 56 longitudinal magnetic resonance imaging studies. Hum Brain Mapp 2012; 33(8): 1987-2002.
- Marrie RA, et al: A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: overview. Mult Scler 2015; 21(3): 263-281.
- Murtonen A, et al: Common comorbidities and survival in MS: Risk for stroke, type 1 diabetes and infections. Mult Scler Relat Disord 2018; 19: 109-114.
- Thormann A, et al: Comorbidity in multiple sclerosis is associated with diagnostic delays and increased mortality. Neurology 2017; 89(16): 1668-1675.
- Koch-Henriksen N, Magyari M, Laursen B: Registries of multiple sclerosis in Denmark. Acta Neurol Scand 2015; 132(199): 4-10. doi: 10.1111/ane.12424.
- Koch-Henriksen N, et al: Incidence of MS has increased markedly over six decades in Denmark particularly with late onset and in women. Neurology 2018; 0:e1-e10.
InFo NEUROLOGY & PSYCHIATRY 2019; 17(4): 20-22 (published 6/20/19; ahead of print).