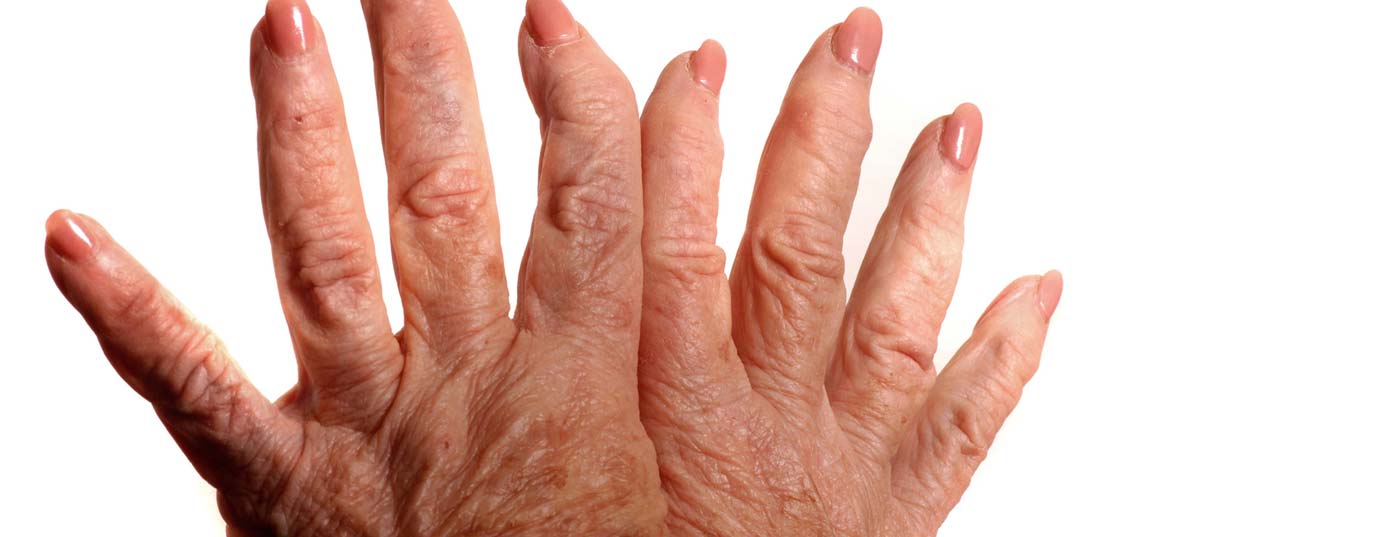
Prof. Paul Hasler, MD, Chief of Rheumatology, Kantonsspital Aarau, discussed the new approval of baricitinib in the indication of moderate to severe active RA with HAUSARZT PRAXIS. Where does the new active ingredient fit into the already broad spectrum of therapies? In addition, news from this year’s EULAR congress and research that is likely to keep the scientific community busy in the future were discussed.
Prof. Dr. Hasler, what is the current therapeutic standard in the field of rheumatoid arthritis (RA)? How does baricitinib, a Janus kinase inhibitor recently approved in this indication, fit into the therapeutic spectrum and with which therapeutic regimen is it used?
Prof. Dr. Hasler:
The revised ACR/EULAR criteria of 2010 [1] are used for diagnosis and classification. These aim to give you relatively high sensitivity and specificity even in early arthritis – yet diagnostic acuity naturally decreases in early stages compared with later ones. As a general rule, the sooner you start treatment, the better. So the goal is to detect, diagnose and treat the disease as early as possible.
According to the EULAR algorithm [2], one starts first with the standard preparation methotrexate (MTX) or comparable basic therapeutics such as leflunomide, less sulfasalazine or hydroxychloroquine. Regarding the combination of these agents, there are different preferences among rheumatologists, e.g. in severe RA a triple combination of MTX, sulfasalazine and hydroxychloroquine is possible (if necessary combined with prednisone).
However one proceeds: After three months of initial therapy, a good response should already be present. The achievement of the actual therapeutic goal, i.e. “no disease activity” (i.e. “remission”) is to be assessed after six months. Depending on the case, the goal may also be, in agreement with the patient, maximum improvement, i.e., the lowest possible disease activity with tolerable therapy (if remission seems unlikely).
If the therapy goal is missed, an alternative must be sought. If the first basic therapy has had an insufficient effect or has not been tolerated, a combination or a change can be made. There is also the option of using a biologic or even a JAK inhibitor [2].
Whenever possible, MTX continues to be used concomitantly, as it significantly enhances the effect especially with TNF inhibitors, co-stimulation inhibition with abatacept, and anti-B-cell therapy with rituximab. Although this is less pronounced with JAK inhibitors and with IL inhibition with tocilizumab, combination with MTX is also recommended. Patients who previously tolerated MTX well but in whom it had an insufficient effect may benefit from such a combination. If MTX is not tolerated, for example, tocilizumab or baricitinib can be used as monotherapy. Here, the data are significantly better than for monotherapy with the traditional TNF inhibitors (although registries show that a substantial percentage of patients also respond well with TNF inhibitor monotherapy).
Which patients will benefit most from the new approval?
On the one hand, patients who do not want any injections or no further injections in addition to MTX, i.e. who prefer oral therapy (once daily in the case of baricitinib). On the other hand, those who have not improved sufficiently with MTX or who have not responded to biologics (or the JAK inhibitor tofacitinib, which has been approved for some time).
The usual dose of baricitinib is 4 mg/d. In renal insufficiency with clearance between 30 and 60 ml/min/1.73 m2 or if the patient is at risk of infection (chronic or recurrent infections), the reduced dose of 2 mg/d is recommended. In addition, even with prolonged remission at the higher dose (plus perhaps concomitant MTX), one may consider lowering the dose to 2 mg/d on a trial basis. After all, as is the case everywhere in medicine, patient compliance is a very decisive factor for the success of the therapy.
What is the principle of action and the clinical study situation in the field of Janus kinase inhibitors (or what other representatives of this class of agents exist)?
The effect on hematopoiesis, inflammation and immune function is due to inhibition of Janus kinases (there are four of them, JAK 1, 2, 3 and TYK 2). In addition to RA, JAK inhibitors are also being investigated in other diseases that respond to these mechanisms of action – from psoriasis and psoriatic arthritis to seronegative spondyloarthritis and the collagenoses.
Another Janus kinase inhibitor is tofacitinib. Baricitinib and tofacitinib are excreted renally, and tofacitinib is also metabolized hepatically. Personally, I would check blood formation and liver values in both representatives (among other things to check whether neutrophil granulocytes are reduced). Headache is reported to be less common with baricitinib than with tofacitinib. The study situation of baricitinib also looks very promising with regard to hepatic side effects.
There are several phase III trials of baricitinib covering key populations of RA. These include patients without or with only low doses of MTX or conventional basic therapeutics [3], and those who, in addition to MTX, also did not respond to or did not tolerate biologics [4,5].
Against which agents was baricitinib compared and in which clinical endpoints did it show success?
Baricitinib was compared against placebo, MTX, the TNF inhibitor adalimumab, and after failure of multiple biologics of different mechanisms of action [3–5]. The primary endpoint in each case was ACR20 response. Some data: For example, 4-mg baricitinib was superior to MTX monotherapy at 24 weeks (77 vs. 62%; p≤0.01) [3]. With conventional DMARDs in the background, it outperformed both placebo (in the 4 mg dose 55 vs. 27%, p<0.001 and 70 vs. 40%, p<0.001) [4,5] and adalimumab (70 vs. 61%, p=0.014) [4] after twelve weeks. In addition, benefits were found in several secondary endpoints, such as DAS28-CRP.
How do you assess the safety profile?
I consider the safety profile to be good, and there are no decisive safety signals that would make you sit up and take notice. Of course, long-term data from currently ongoing extension studies will need to be followed up. It applies to all preparations: especially with regard to infections that can break out suddenly and be severe, you need to be very vigilant. Diverticulitis and perforations may occur, especially in the older RA population. Vaccinations should be kept up to date, and the annual flu shot is recommended. If zoster occurs, antiviral therapy must be initiated immediately (i.e., patients must be appropriately informed so that they can react quickly even if prodromes occur).
To date, there is no evidence that the lipid parameter increases seen with other RA agents such as tofacitinib and the anti-TNFs occur with increased major adverse cardiac events (“MACE”). Lipid parameters should be determined appropriately, and patients should be treated according to guidelines for hyperlipidemia.
Were there any other important findings in the field of rheumatoid arthritis at the 2017 EULAR Congress, which was held in Madrid in June?
For example, the state of research in the field of biosimilars was discussed, which, like biologics, have now undergone major development. However, their introduction is currently still rather tentative; they must first prove themselves in everyday clinical practice and in post-marketing studies. To date, there are still limited data on switching from an originator drug to a corresponding biosimilar – the so-called NOR-SWITCH study [6] was the topic of discussion at the congress, and others will follow. Non-inferiority margin was defined as 15% in NOR-SWITCH. The question now is: If the disease is only just controlled with the originator drug, do we lose control with the biosimilar at the same dose?
In addition, there are other JAK inhibitors in development, and I expect that in a few years we will have a wide range of these agents available.
Where do you see future fields of research?
Epidemiologically, Prof. Axel Finckh in Geneva, among others, is pursuing interesting approaches investigating the influence of smoking, air pollution, viral/bacterial infections on the development and progression of the disease.
It may also be possible to achieve good treatment results in the future by influencing neutrophil granulocytes. A pioneer in this field of research is Prof. Gerd Burmester from Berlin. Appropriate antibodies against GM-CSF and G-CSF (granulocyte [macrophage] colony stimulating factors) are currently being tested. GM-CSF and G-CSF play a key role in neutrophil activation. The question here will be how to combine these antibodies with other agents such as MTX or leflunomide without putting patients at risk, and how to identify severe courses in advance to justify such therapy.
Interview: Andreas Grossmann
Literature:
- Aletaha D, et al: 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010; 69(9): 1580-1588.
- Smolen JS, et al: EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017; 76(6): 960-977.
- Fleischmann R, et al: Baricitinib, Methotrexate, or Combination in Patients With Rheumatoid Arthritis and No or Limited Prior Disease-Modifying Antirheumatic Drug Treatment. Arthritis & Rheumatology 2017; 69(3): 506-517.
- Taylor PC, et al: Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med 2017; 376: 652-662.
- Genovese MC, et al: Baricitinib in Patients with Refractory Rheumatoid Arthritis. N Engl J Med 2016; 374: 1243-1252.
- Jørgensen KK, et al: Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet 2017; 389(10086): 2304-2316.
HAUSARZT PRAXIS 2017; 12(8): 8-9