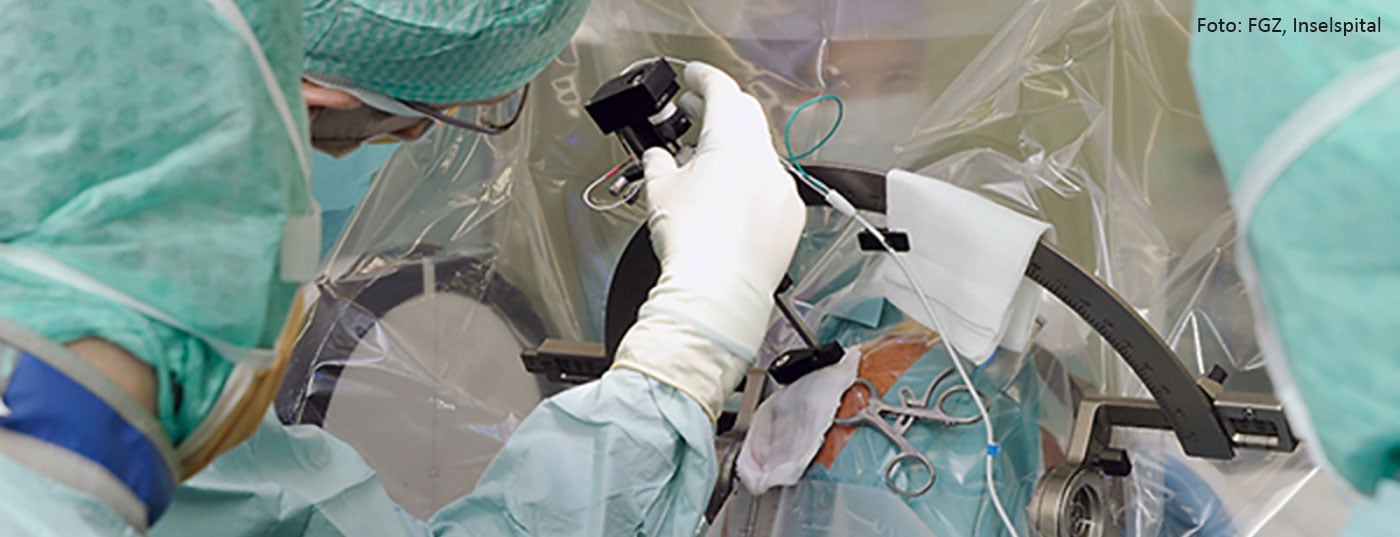
Well over one-third of all epilepsy cases are not adequately treatable with conservative measures, and very many of these patients are suitable for surgical treatment. Diagnostic epilepsy surgery allows precise localization of epileptogenic foci and functional brain areas when conventional diagnostics have reached the limits of their capabilities. Therapeutic epilepsy surgery includes various highly efficient microsurgical, neuromodulatory, and neuroablative procedures. Periprocedural morbidity is rare and operative mortality is the exception. In particular, for the pharmacotherapy-resistant temporal lobe epilepsies, the results of epilepsy surgery have been shown to show the best results with cure of the disease in 60-80%. With appropriate patient selection, careful pre-surgical assessment and execution of the procedure, epilepsy surgery can not only permanently control seizures, but also significantly improve life expectancy and quality of life and significantly reduce treatment costs.
Epilepsies, some of the most common, diverse, and severe neurological disorders, are a diagnostic and therapeutic challenge for any treating physician. Up to 40% of affected patients suffer from persistent seizures despite adequate drug therapy. More than five million epilepsy patients worldwide represent potential candidates for neurosurgical treatment. This review article summarizes the current knowledge on epilepsy surgery and highlights indications, methods, possibilities, risks, and prospects for success of modern neurosurgical epilepsy treatment.
Goals of epilepsy surgery
Surgical epilepsy treatment is a subspecialty of neurosurgery. Epilepsy surgery should be considered to further and invasively clarify seizures that cannot otherwise be adequately diagnosed, as well as to treat conditions that cannot be adequately controlled pharmacologically. The ultimate goal of epilepsy surgery is complete seizure freedom. If this goal is not achievable, the goal is to maximize seizure reduction and thereby minimize epilepsy risks and medication side effects.
Patient selection
In principle, patients with all seizure types and syndromes should first be considered potential epilepsy surgery candidates who are increasingly difficult to treat pharmacologically and for whom at least two antiepileptic drugs at appropriate doses are inadequate. Epilepsy surgery is particularly suitable when side effects of medication already have to be accepted. Preoperatively, several questions should be addressed:
- Are the seizures really of epileptic origin?
- Is the onset focal and unilateral?
- Is it a single focus?
- Where exactly is the focus located?
- Can the excision be complete and without possible neurologic deficits?
Preepilepsy surgical evaluation is complex, multidisciplinary, and should be performed in highly specialized centers, as should the surgery itself. It includes review of previous medical history and a current clinical assessment, neuroradiological examination by magnetic resonance imaging (MRI) according to specific epilepsy protocols and evaluation by an experienced neuroradiologist, recording of seizures by inpatient long-term video electroencephalography (EEG) (telemetry), and neuropsychological and psychiatric assessment. In some cases, special further investigations such as functional imaging and intracranial EEG recordings (so-called pre-epilepsy surgical phase II clarification) are also necessary.
Invasive pre-surgical epilepsy diagnostics.
Delimiting the seizure origin zone (epileptogenic focus) and adjacent eloquent (functionally important) areas as accurately as possible is critical to the performance, safety, and success of epilepsy surgery. If a noninvasive surface EEG (phase I) alone is not sufficiently possible to lateralize and localize the seizure origin zone, an additional, invasive EEG (phase II) can be added as in stereo EEG. This applies in particular when
- even high-resolution imaging could not detect an epileptogenic lesion,
- discrepant results are available regarding the spread of epileptogenic activity in the brain,
- Multifocality must be excluded,
- a narrowly circumscribed resection is indicated to spare adjacent eloquent brain tissue.
Temporary intracerebral depth electrodes and epidural or subdural strip and plate electrodes are stereotactically guided and neuronavigationally inserted via burr hole trephines and craniotomies. In this way, in addition to invasive EEG recording with position determination and delineation of the epileptogenic area, intracranial stimulation can be performed to assess the relation of epileptogenic lesions to eloquent brain areas. The implanted electrodes allow highly sensitive seizure detection, but can only spatially derive excitations of the immediately adjacent brain areas. Due to the increased risk of multiple polyfocal implantations, the electrodes also cannot be inserted in any number and location. Therefore, prudent preoperative planning (making a hypothesis about the location of the seizure origin zone, determining the type, number, and location of electrodes) and careful performance of the implantation itself are critical.
Usually, at least two patient-typical seizures must be recorded before the decision to perform therapeutic epilepsy surgery can be made. Typically, the electrodes, which are often inserted only via burr hole trephines, are removed after invasive monitoring is completed, and the therapeutic procedure is performed in a second session, incorporating any previous incisions and trephines. If strip or plate electrodes have been implanted via craniotomies, therapeutic epilepsy surgery can be performed at the same time as explantation to spare the patient further unnecessary anesthesia and surgery.
Diagnostic epilepsy surgery is associated with temporary morbidity of about 8%, and permanent morbidity is very rare at 0.6%. Mortalities are not usually expected. The use of parenchymal electrodes instead of epidural or subdural electrodes can minimize the risk. Seizure origins are reliably detected in over 99% of cases, so the benefits of invasive monitoring outweigh the potential risks. Diagnostic epilepsy surgery leads to therapeutic epilepsy surgery intervention in 95%.
Classification of surgical epilepsy therapy.
Therapeutic epilepsy surgery procedures include various microsurgical, stereotactic, and functional techniques. In principle, interventions can be divided clinically into curative and palliative and methodologically into resective, disconnective, destructive, neuromodulative, and neuroablative procedures. Resective and destructive procedures eliminate or destroy epileptogenic areas, and disconnective isolative and neurostimulatory functional procedures disrupt or modulate epileptogenic networks. Procedures with curative goals include resections, lesionectomies, and hemispheric interventions, whereas callosotomies, multiple subpial transections, and neuromodulatory procedures have palliative goals. Neuroablative techniques are an exception and are used in both curative and palliative therapeutic approaches.
Anatomically, about two-thirds of the operations are performed temporally, all others extratemporally or multifocally and combined.
Furthermore, procedures are differentiated according to the degree of surgical invasiveness. Thus, hemispherectomies are the most invasive and peripheral nerve stimulations are the least invasive. Some neuroablative ultrasound-guided and radiosurgery procedures do not involve surgical invasiveness. Several factors favor the chance of seizure control by epilepsy surgery; these include containability of the lesion, completeness of elimination, absence of bilateral tonic-clonic seizures, and early timing of surgery.
Resective epilepsy surgery
The resective epilepsy surgical procedures are mostly temporal and have primarily a curative therapeutic approach. Patients with focal or regional seizure origin are usually suitable. The epileptogenic brain tissue to be excised may be variably localized, uni- or multifocally located, and may range in size from a few millimeters to an entire hemisphere. The epileptogenic zones should be sufficiently circumscribed and not located in an eloquent brain area so that complete and safe removal is possible and can lead to complete seizure freedom. Resective procedures include temporal, extratemporal, and neocortical resections, lesionectomies, and hemispheric procedures.
Temporal resections
Temporal lobectomies with extensive removal of the temporal lobe including the mesial structures are very rare nowadays. In contrast, anterior temporal lobectomy or temporal lobe resection with its different variations is the most common procedure in epilepsy surgery with 70-80%. While originally two-thirds resections of the temporal lobe were performed with or without amygdalohippocampectomies (corticoamygdalohippocampectomies), there are now different, less resective modifications such as the anterior partial lobectomies, hippocampectomies, cortical and selective amygdalohippocampectomies, corticoamygdalectomies, topectomies, or multiple hippocampal transections performed in isolation or in combination. Overall, the prospects of seizure freedom are high, ranging from 60-80%.
Extratemporal resections
Extratemporal resections include solitary lobectomies, multilobectomies, hemispherectomies, hemispherotomies, lesionectomies, and topectomies. Success rates are lower overall than with temporal interventions, with seizure freedom ranging from 30 to 60%. After temporal lobe resections, frontal lobe procedures are the most common resective procedures, followed by parietal lobe surgery and occipital lobe resections. The rates of seizure freedom are over 45% in each case.
Neocortical resections
Procedures on the neocortex include temporal as well as extratemporal operations in the area of one or more brain lobes. Depending on the underlying lesion, large resections may be necessary to remove the epileptogenic zones, and the extent should take into account the relation to eloquent areas and the risk for functional deficits. Neocortical resections also include topectomies, circumscribed resections of precisely localized epileptogenic areas within a lobe of the brain. Depending on the localization, seizure freedom is described in about 60% of patients.
Lesionectomies
Lesional epilepsies are characterized by epileptogenic structural lesions such as vascular malformations, focal cortical dysplasias, mesial temporal scleroses, encephalomalacias, and low-grade neoplasms. Their removal leads to seizure freedom in 70-90% depending on the completeness of the resection of the epileptogenic area. Multifocal and eloquently located lesions can often only be excised incompletely, and interventions are associated with correspondingly lower success rates. Depending on the underlying pathology, adjuvant neuroradiological, oncological and radiooncological procedures are used in addition.
Hemispheric Interventions
Hemisphere surgery includes hemispherectomies, hemidecortications, and hemisphererotomies and their respective variations. They are among the most effective, but also the most invasive and radical epilepsy surgical procedures. Candidates are predominantly children with extensive hemispheric brain damage and lateralized epilepsy. Underlying pathologies include porencephalic cysts, hemiatrophies, hemimegaloencephalies, Rasmussen’s encephalitides, Sturge-Weber angiomas, or severe traumatic lesions.
Early classic anatomic hemispherectomies were associated with high complication rates and are usually no longer used. Modifications such as functional Rasmussen’s hemispherectomy are far less resective and predominantly disconnective; they involve removal of the temporal lobe and central cortex with uncoupling of the frontal and occipitotemporal neocortex from the subcortical structures and corpus callosum, which nevertheless remain anatomically intact. Functional hemispherotomies represent further technical refinement and include only very limited resections of brain tissue. The epileptogenic ipsilateral hemisphere is largely disconnected from the subcortical centers and contralateral hemisphere but is anatomically preserved.
After hemispheric surgery, further seizures are absent in over 73% of patients; seizure freedom can be achieved in 70-80% of children and up to 90% of adults.
Non-resective epilepsy surgery.
The nonresective, isolative, and functional epilepsy surgical procedures are performed far less frequently, are usually less promising, and usually have a palliative therapeutic approach. The goal is to reduce the severity of the epilepsy disorder in question. The prospects of complete seizure freedom are low, but seizure frequency can be significantly reduced in about 50% of patients. Non-resective surgeries include disconnective and ablative procedures and neuromodulatory implants; they are considered when resective interventions are not practical or possible.
Callosotomies: Callosotomies with partial or complete transection of the corpus callosum are mostly performed in children. Patients with extensive synchronous bihemispheric seizure origin without identifiable resectable focus are possible candidates. The goal is to prevent spread of epileptogenic activity across the bar to the opposite hemisphere. Main indications are usually otherwise untreatable atonic fall attacks, Lennox-Gastaut syndromes, and multifocal epilepsies.
The procedure is usually performed in one or two steps. In an initial surgical session, the anterior two-thirds of the bar is transected to minimize the risk of disconnection syndrome. If this is not sufficient, the entire corpus callosum can be severed in a second intervention. Depending on the type and severity of epilepsy, seizure freedom can be expected in 5-35% and a significant reduction in seizure frequency in 60-65%.
Multiple subpial transections: If the epileptogenic area is located in a functionally important brain region, multiple subpial transections are an option for palliative treatment of seizure onset and propagation within eloquent areas. Transections consist of vertical neocortical incisions in the gray matter or hippocampus with the goal of preserving the anatomic function of the region and suppressing seizure onset. They are usually performed together with resective surgery.
After transections alone, a greater than 95% reduction in seizure frequency is described in 60-70% of patients, depending on seizure type. If transections are performed together with resections, success rates of 70-90% can be achieved.
Neuromodulation
Implantable augmentative neuromodulatory therapy options for the treatment of primarily multifocal epilepsies and severe disease courses include direct neurostimulation of central brain structures via continuous deep and responsive superficial brain stimulation and indirect neurostimulation via peripheral nerves such as the vagi and trigeminal nerves. Non-implantable noninvasive procedures such as transcranial magnetic stimulation and direct electrical stimulation are only conditionally related to epilepsy surgery and will therefore not be discussed in detail here. The respective mechanisms of action of the processes have not yet been fully clarified. Their modes include continuous and periodic seizure-independent (“open-loop”) and intermittent seizure-associated (“closed-loop”) stimulation.
Deep brain stimulation: Deep brain stimulation with insertion of electrodes into profound core areas for chronic stimulation via neuropacemakers is used as a palliative therapy option. Electrode implantations are performed either directly into the epileptogenic zone or distantly into circumscribed nuclear areas such as the hippocampus, cerebellum, and thalamus. Rare alternative targets include substantia nigra, locus coeruleus, and subthalamic and caudate nuclei. Stimulation is usually continuous and seizure-independent, although approaches to adaptive, seizure-linked stimulation exist. Freedom from seizures is not usually expected with this treatment. However, depending on the site of action of the stimulation, reductions in seizure frequency have been described in 15% (hippocampus), 20% (cortex), and 26% (thalamus) of patients.
Superficial brain stimulation: Superficial brain stimulation is performed in patients with no more than two epileptogenic foci using electrodes implanted subdurally and parenchymatously near the epileptogenic area. During responsive cortical stimulations, seizures are dedected and a limited cortex area is stimulated via a neurostimulator and an appropriate algorithm. This leads to the termination of the ictal discharges. Approximately 38% of patients achieve a seizure reduction of at least 50% as a result.
Vagus nerve stimulation: Stimulation of the vagus nerve is indicated in epilepsy patients in whom no circumscribed, surgically accessible epileptogenic areas can be identified and for whom resective procedures are not suitable. Electrodes are applied to the left vagus nerve in the neck and connected to a neurostimulator. Pacing can be intermittent or ECG-induced by impending seizures and a combination of both modes. Seizure freedom is found in an average of only 14% of treated patients, but seizure frequency can be reduced in about 51%.
Trigeminal nerve stimulation: In this procedure, branches of the trigeminal nerve are stimulated transcutaneously or invasively (the ophthalmic, supraorbital, and infraorbital nerves on both sides, respectively). So far, only a few results are available on this new method. Sustained reductions in seizure frequency of greater than 50% were documented in 44-59% of patients.
Neuroablation
Neuroablative treatment methods occupy a special position in epilepsy surgery. In their therapeutic approach, they are both curative and palliative, cause permanent lesions, are used in particular to treat mesial temporal lobe epilepsies as well as circumscribed deep-seated epileptogenic foci, and include thermocoagulative procedures (including MRI-guided methods such as focused ultrasound and laser ablation) and stereotactic-assisted procedures (radiofrequency ablation and radiosurgery). Seizure freedom of up to 25% (radiofrequency ablation), 77% (radiosurgery), and 86% (laser ablation) has been described, depending on the type of epilepsy and the procedure. For the novel ultrasound treatment, conclusive study results with data on seizure frequency remain to be seen.
Advantages of these procedures are minimal invasiveness as in the case of radiofrequency and laser ablation or even lack of surgical invasiveness as in the case of ultrasound ablation and radiosurgery. Experience and results to date are still limited and inconsistent. Therefore, a conclusive assessment is not possible at present. Comparable success rates to resective series have been achieved with radiosurgery, but it must be remembered that results are not established until a latency of many months, during which time cerebral edema requiring treatment often occurs and symptoms may increase, necessitating protracted steroid therapy with its attendant side effects.
Possibilities, risks and prospects of success
Epilepsy surgery complications are rare, usually not severe, and only temporary. They include the general risks of an intervention, such as infections and bleeding, as well as specific risks, depending on the type and extent of the intervention and the particular location. Complication rates for all procedures combined are 5-11% overall. Resective epilepsy surgery is associated with a temporary morbidity of about 5% and a permanent morbidity of 1.5%. Perioperative mortality is low, ranging from 0.1 to 0.5% overall. For temporal procedures, perioperative mortality is 0.4%; for extratemporal procedures, it is 1.2%. Transient neurologic complications are more common in children and after extratemporal surgery and occur in up to approximately 11%. Persistent deficits are found in only about 5%. Reoperations due to complications or to treat postoperative persistent seizures are rarely necessary. However, with appropriate care in the indication, second operations for further epilepsy treatment can be performed safely and effectively if the initial operation was not sufficiently effective or was unsuccessful.
The chances of success of therapeutic epilepsy surgery depend on the type of epilepsy, underlying pathology, location of the epileptogenic zone, exactness of localization, and completeness of resection. On average, the seizure-free rate after all interventions was 62.5%. Temporal epilepsies account for up to 76% and extratemporal epilepsies for 34-56%. In long-term studies, sustained seizure freedom is found after a total of 48% of all epilepsy surgery cases. The chances of success are comparable in children and adults. Resections for hippocampal sclerosis and benign and low-grade tumors show more frequent seizure freedom compared with all other epilepsy-causing entities. In addition, generally more favorable courses are found after lesional epilepsy surgery if epileptogenic abnormalities could be detected preoperatively by image morphology.
Neuroprotection through epilepsy surgery.
Epilepsies with chronic uncontrollable seizures are clinically malignancies. While seizure-free epilepsy patients have a comparable life expectancy to the general population, patients with persistent seizures have a 4.7% increase in mortality. In addition, inadequately treated epilepsies may be associated with the risk of progressive cortical atrophy and cognitive decline. Side effects of long-term drug therapy are also common.
Studies have shown that epilepsy surgery can increase both life expectancy and quality of life for affected patients and reduce treatment costs. Seizure freedom, especially at a young age, reduces the risk of cognitive, behavioral, and psychological problems and improves social integration. If the epileptogenic focus can be precisely narrowed preoperatively and completely removed intraoperatively with low risk, neurosurgical treatment for pharmacotherapy-resistant epilepsy is more likely to result in seizure freedom than any further conservative treatment attempt. By preventing progressive neurological damage, epilepsy surgery is neuroprotective, and the risk of injury or even death is reduced.
Superior effect, but too rarely used
Epilepsy surgery has evolved immensely over the past decades and now includes various neuromodulatory and neuroablative options in addition to the classic resective and disconnective microsurgical procedures. In the implementation of surgical epilepsy therapy, various modern imaging, stereotactic and electrophysiological principles as well as special methods are used, e.g. neuronavigation, brain mapping, radiofrequency, laser technology, ultrasound and radiosurgery. With careful preoperative interdisciplinary assessment, patient selection, and indication in a specialized center, the chances of success with epilepsy surgery are high and the risks are low. Although the efficacy and superiority of surgical over drug therapy in the treatment of pharmacotherapy-resistant epilepsies is well established, epilepsy surgery is still considered too rarely and too late as a therapeutic option.
Literature:
- Barbaro NM, et al: A multicenter, prospective pilot study of gamma knife radiosurgery for mesial temporal lobe epilepsy: seizure response, adverse events, and verbal memory. Ann Neurol 2009; 65(2): 167-175.
- Bergey GK, et al: Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology 2015; 84(8): 810-817.
- Connor DE Jr, et al: Vagal nerve stimulation for the treatment of medically refractory epilepsy: a review of the current literature. Neurosurg Focus 2012; 32(3): E12.
- DeGiorgio CM, et al: Trigeminal nerve stimulation for epilepsy: long-term feasibility and efficacy. Neurology 2009; 72(10): 936-938.
- DeGiorgio CM, et al: Pilot study of trigeminal nerve stimulation (TNS) for epilepsy: a proof-of-concept trial. Epilepsia 2006; 47(7): 1213-1215.
- Engel J Jr, et al: Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA 2012; 307(9): 922-930.
- Engel J Jr, et al: Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology 2003; 60(4): 538-547.
- Englot DJ, Chang EF, Auguste KI. Vagus nerve stimulation for epilepsy: a meta-analysis of efficacy and predictors of response. J Neurosurg 2011; 115(6): 1248-1255.
- Fisher R, et al: Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 2010; 51(5): 899-908.
- Griessenauer CJ, et al: Hemispherectomy for treatment of refractory epilepsy in the pediatric age group: a systematic review. J Neurosurg Pediatr 2015; 15(1): 34-44.
- Guénot M, et al: SEEG-guided RF-thermocoagulation of epileptic foci: a therapeutic alternative for drug-resistant non-operable partial epilepsies. Adv Tech Stand Neurosurg 2011; 36: 61-78.
- Guerrini R, Rosati A, Giordano F, Genitori L, Barba C. The medical and surgical treatment of tumoral seizures: current and future perspectives. Epilepsia 2013; 54 Suppl 9: 84-90.
- Hader WJ, et al: Complications of epilepsy surgery: a systematic review of focal surgical resections and invasive EEG monitoring. Epilepsia 2013; 54(5): 840-847.
- Heck CN, et al: Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia 2014; 55(3): 432-441.
- Hu WH, et al: Hemispheric surgery for refractory epilepsy: a systematic review and meta-analysis with emphasis on seizure predictors and outcomes. J Neurosurg 2015 Oct 23: 1-10 [Epub ahead of print].
- Jobst BC, Cascino GD. Resective epilepsy surgery for drug-resistant focal epilepsy: a review. JAMA 2015; 313(3): 285-293.
- Jobst BC, Darcey TM, Thadani VM, Roberts DW. Brain stimulation for the treatment of epilepsy. Epilepsia 2010; 51 Suppl 3: 88-92.
- Jolesz FA, McDannold NJ. Magnetic resonance-guided focused ultrasound: a new technology for clinical neurosciences. Neurol Clin 2014; 32(1): 253-269.
- Krishnaiah B, Ramaratnam S, Ranganathan LN. Subpial transection surgery for epilepsy. Cochrane Database Syst Rev 2015; 12: CD008153.
- Kuzniecky R, Devinsky O. Surgery Insight: surgical management of epilepsy. Nat Clin Pract Neurol 2007; 3(12): 673-681.
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med 2000; 342(5): 314-319.
- Laxpati NG, Kasoff WS, Gross RE. Deep brain stimulation for the treatment of epilepsy: circuits, targets, and trials. Neurotherapeutics 2014; 11(3): 508-526.
- Lega BC, Halpern CH, Jaggi JL, Baltuch GH. Deep brain stimulation in the treatment of refractory epilepsy: update on current data and future directions. Neurobiol Dis 2010; 38(3): 354-360.
- Lew SM. Hemispherectomy in the treatment of seizures: a review. Transl Pediatr 2014; 3(3): 208-217.
- Lhatoo SD, Moghimi N, Schuele S. Tumor-related epilepsy and epilepsy surgery. Epilepsia 2013; 54 Suppl 9: 1-4.
- Liu SY, et al: Clinical outcomes and quality of life following surgical treatment for refractory epilepsy: a systematic review and meta-analysis. Medicine (Baltimore) 2015; 94(6): e500.
- McIntosh AM, Kalnins RM, Mitchell LA, Fabinyi GC, Briellmann RS, Berkovic SF. Temporal lobectomy: long-term seizure outcome, late recurrence and risks for seizure recurrence. Brain 2004; 127(Pt 9): 2018-2030.
- Monteith S, et al: Transcranial magnetic resonance-guided focused ultrasound for temporal lobe epilepsy: a laboratory feasibility study. J Neurosurg 2016 Feb 12: 1-8 [Epub ahead of print].
- Morrell F, Whisler WW, Bleck TP. Multiple subpial transection: a new approach to the surgical treatment of focal epilepsy. J Neurosurg 1989; 70(2): 231-239.
- Morrell M. Brain stimulation for epilepsy: can scheduled or responsive neurostimulation stop seizures? Curr Opin Neurol 2006; 19(2): 164-168.
- Morrell MJ, Halpern C. Responsive direct brain stimulation for epilepsy. Neurosurg Clin N Am 2016; 27(1): 111-1121.
- Morrell MJ; RNS System in Epilepsy Study Group. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 2011; 77(13): 1295-1304.
- Nair DR, Burgess R, McIntyre CC, Lueders H. Chronic subdural electrodes in the management of epilepsy. Clin Neurophysiol 2008; 119(1): 11-28.
- Nair DR. Management of drug-resistant epilepsy. Continuum (Minneap Minn) 2016; 22(1 Epilepsy): 157-172.
- Nowell M, Miserocchi A, McEvoy AW, Duncan JS. Advances in epilepsy surgery. J Neurol Neurosurg Psychiatry 2014; 85(11): 1273-1279.
- Nowell M, Miserocchi A, McEvoy AW. Tumors in epilepsy. Semin Neurol 2015; 35(3): 209-217.
- Panebianco M, Rigby A, Weston J, Marson AG. Vagus nerve stimulation for partial seizures. Cochrane Database Syst Rev 2015; 4: CD002896.
- Patil AA, Andrews R. Long term follow-up after multiple hippocampal transection (MHT). Seizure 2013; 22(9): 731-734.
- Patil AA, Chamczuk AJ, Andrews RV. Hippocampal transections for epilepsy. Neurosurg Clin N Am 2016; 27(1): 19-25.
- Quigg M, Harden C. Minimally invasive techniques for epilepsy surgery: stereotactic radiosurgery and other technologies. J Neurosurg 2014; 121 Suppl: 232-240.
- Ryvlin P, Cross JH, Rheims S. Epilepsy surgery in children and adults. Lancet Neurol 2014; 13(11): 1114-1126.
- Ryvlin P, Kahane P. Does epilepsy surgery lower the mortality of drug-resistant epilepsy? Epilepsy Res 2003; 56(2-3): 105-120.
- Salanova V, et al: Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology 2015; 84(10): 1017-1025.
- Sawhney IM, Robertson IJ, Polkey CE, Binnie CD, Elwes RD. Multiple subpial transection: a review of 21 cases. J Neurol Neurosurg Psychiatry 1995; 58(3): 344-349.
- Schramm J. Epilepsy surgery and the evolution of clinical and translational science. Neurosurgery 2014; 61 Suppl 1: 54-65.
- Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol 2008; 7(6): 525-537.
- Sprengers M, Vonck K, Carrette E, Marson AG, Boon P. Deep brain and cortical stimulation for epilepsy. Cochrane Database Syst Rev 2014; 6: CD008497.
- Surges R, Elger CE. Reoperation after failed resective epilepsy surgery. Seizure 2013; 22(7): 493-501.
- Tanriverdi T, et al: Morbidity in epilepsy surgery: an experience based on 2449 epilepsy surgery procedures from a single institution. J Neurosurg 2009; 110(6): 1111-1123.
- Téllez-Zenteno JF, et al: Long-term outcomes in epilepsy surgery: antiepileptic drugs, mortality, cognitive and psychosocial aspects. Brain 2007; 130(Pt 2): 334-345.
- Téllez-Zenteno JF, et al: Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res 2010; 89(2-3): 310-318.
- West S, Nolan SJ, Cotton J, Gandhi S, Weston J, Sudan A, Ramirez R, Newton R. Surgery for epilepsy. Cochrane Database Syst Rev 2015; 7: CD010541.
- Wiebe S, et al: Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 2001; 345(5): 311-318.
- Wiebe S, Jetté N. Epilepsy surgery utilization: who, when, where, and why? Curr Opin Neurol 2012; 25(2): 187-193.
- Wu C, Sharan AD. Neurostimulation for the treatment of epilepsy: a review of current surgical interventions. Neuromodulation 2013; 16(1): 10-24.
InFo NEUROLOGY & PSYCHIATRY 2016; 14(3): 12-17.