In patients with suspected pulmonary embolism, risk stratification is important because it guides all subsequent therapeutic decisions. High-risk patients with hemodynamic instability must be identified promptly so that systemic thrombolytic therapy can be initiated immediately if there are no contraindications (grade 1B). Catheter (grade 2aC) or surgical (grade 1C) embolectomy is recommended for high-risk patients with contraindication to or failure of systemic thrombolysis. Systemic thrombolysis should not be used routinely in the absence of high-risk situations (grade 3B). Thrombolysis (grade 2aB) or catheter-assisted thrombolysis (grade 2bB) may be considered in patients at intermediate-high risk without hemodynamic instability, especially if clinical deterioration occurs with anticoagulation alone.
Pulmonary embolism is a life-threatening condition with mortality of up to 17% in the first three months [1]. In this initial phase, survival depends primarily on hemodynamic status and right ventricular dysfunction. It is therefore of importance to promptly identify those patients who are particularly at risk. This risk stratification will be highlighted in this article. Accordingly, depending on the risk group, there are also recommendations regarding therapy, whether systemic thrombolysis, catheter-based treatment, or surgical embolectomy is indicated. These specific treatment strategies for each risk group are also discussed in this article. The article is based on the current recommendations of the 2014 ESC guidelines [2].
Risk stratification
First, it is important to identify those patients who are at high risk for mortality, especially in the early phase of pulmonary embolism (Fig. 1) . The mortality risk for these high-risk patients is estimated to be as high as 24.5% [3,4].
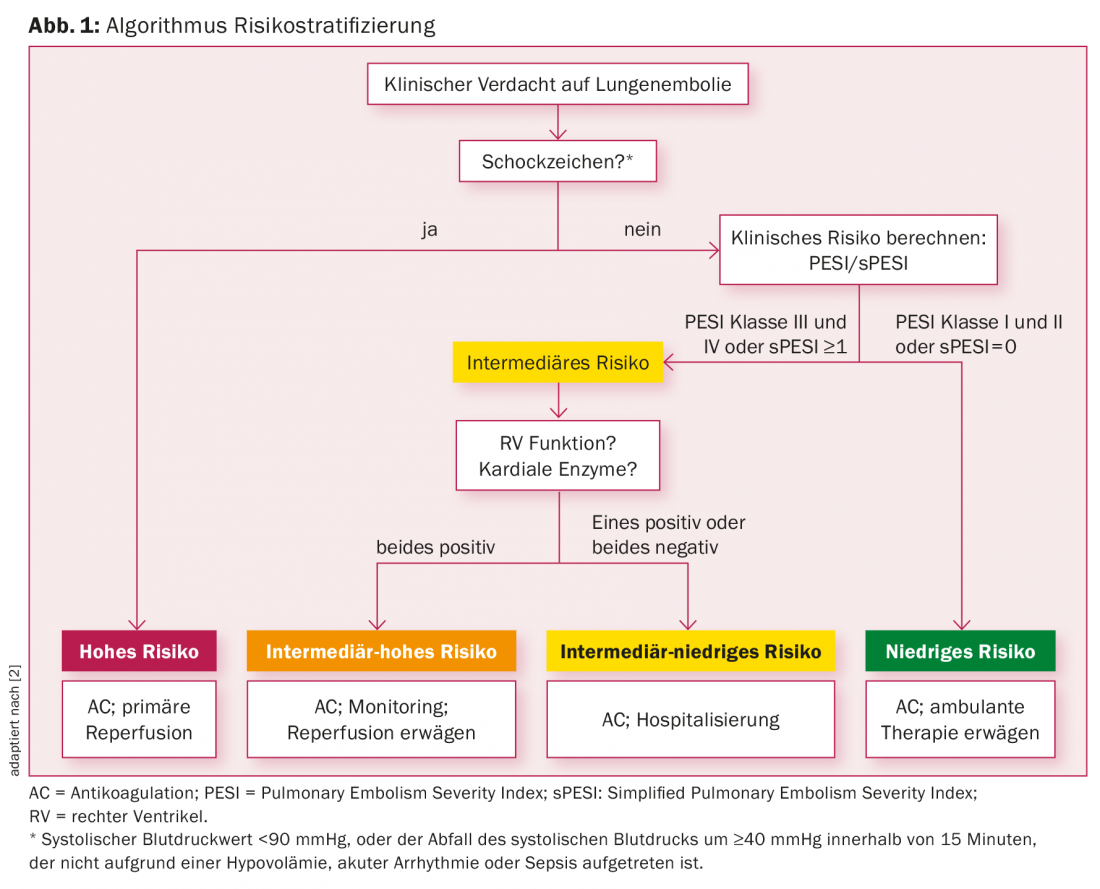
Patients are identified as high-risk if they have signs of shock or hypotension, defined as a systolic blood pressure value <90 mmHg, or the drop in systolic blood pressure by ≥40 mmHg within 15 minutes (not due to hypovolemia, arrhythmia, sepsis).
General therapy
All patients should receive anticoagulation promptly, regardless of their risk situation, with the aim of reducing mortality on the one hand and the risk of recurrence of venous thromboembolism on the other. The standard duration of anticoagulation is at least three months.
Conventional anticoagulation begins with immediate therapy with parenteral anticoagulation (heparins), later overlapping with vitamin K antagonists.
Phase III studies are available for all new oral anticoagulants (DOAK: rivaroxaban, edoxaban, dabigatran, and apixaban), all of which are non-inferior to vitamin K antagonists in terms of efficacy. It is possible that DOACs may even be considered safer in terms of relevant bleeding [5]. All substances are approved in the European Union for the treatment of pulmonary embolism.
Because anticoagulation alone shows little direct effectiveness on right ventricular function, the additional therapeutic approaches discussed below (systemic thrombolysis, catheter-based thrombolytic therapy, surgical embolectomy) must be evaluated for high and intermediate risk.
Therapy of the high-risk patients
High-risk patients need accelerated (fast-track) follow-up. In these patients, the primary recommendation according to ESC guidelines is to perform systemic thrombolytic therapy (evidence level 1B).
Systemic thrombolysis showed the greatest benefit within the first 48 hours after symptom onset. There was improvement in clinical and echocardiographic findings in the first three days after thrombolysis in more than 90% of cases [6,7]. The large randomized Peitho trial [8] confirmed the effectiveness of systemic thrombolysis even in hemodynamically stable intermediate-risk patients. A reduction in hemodynamic collapse and mortality (2.6%) was observed in patients receiving systemic thrombolysis compared with patients receiving anticoagulation alone (5.6%). However, there was an increase in “major” bleeding in the thrombolysis group: 6.3% vs. 1.5% in the placebo group. Further, there was a significant increase in hemorrhage-related cerebrovascular insults 2% vs. 0.2%. Against this background, systemic thrombolysis is used cautiously at many centers.
If contraindications to systemic thrombolysis are present, catheter-based interventional thrombolysis (grade 2aC) should be weighed against surgical pulmonary embolectomy (grade 1C). The same applies if primary thrombolytic therapy has not yet led to the desired success.
To date, the evidence is unclear as to whether surgical or catheter-based thrombolysis results in better patient outcomes in those patients specifically at risk. To clarify this issue, an interdisciplinary randomized trial (special trial) is currently being conducted at Inselspital in Bern, in which intermediate-high-risk patients and high-risk patients are randomly assigned to a treatment arm (surgical embolectomy or catheter-based thrombolysis). First results are expected in 2017.
Therapy of intermediate and low-risk patients.
If there are no signs of shock or hypotension in suspected pulmonary embolism, it is an intermediate, resp. a low-risk situation. To differentiate the intermediate from the low risk group, a clinical decision tool helps further, the so-called Pulmonary Embolism Severity Index (PESI) or the Simplified Pulmonary Embolism Score (sPESI), i.e. the simplified version of the same score [3]. Table 1 provides an overview of the criteria of the sPESI. Each of the above criteria results in one point if present. If the sum of the points is zero, the patient is a low-risk patient. These patients receive only anticoagulation without reperfusion therapy. In addition, low-risk patients will be evaluated to determine whether they can be treated as outpatients.
Accordingly, patients with an sPESI score of ≥1 point are classified as intermediate-risk patients. Intermediate-risk patients require further diagnostic workup to assess right ventricular function due to pressure loading from pulmonary embolism. Patients who have right ventricular dysfunction on echocardiography or CT angiography and elevated cardiac enzymes (positive cardiac troponin) are placed in the intermediate-high risk category. These patients must be monitored in an intermediate care unit for the first 24 hours (Grade 1B). Primary revascularization treatment is not mandatory. In these patients, thrombolytic therapy should be considered if clinical deterioration occurs or clinical situation does not improve with initial anticoagulation (grade 2aB). If contraindications to thrombolysis exist, catheter-based interventional (grade 2bB) or surgical embolectomy (grade 2bC) may be considered as an alternative.

The interventional catheter-based therapy
Catheter-based intervention aims to reduce thrombus burden in the central pulmonary arteries or in the lower lobe arteries. This reduces right ventricular strain, leading to improvement in symptoms and survival [9].
On the one hand, conventional catheter-based intervention techniques exist for those patients who have an absolute contraindication to thrombolysis [10].
The thrombus can be removed catheter-technically either by fragmentation using a pigtail catheter or rheolytically using pressure gradients (e.g. angio-jet catheter) or aspiration-technically (e.g. Argon Medical Device) or finally rotation-technically (e.g. Aspirex catheter).
In addition, there are catheter-assisted locoregional thrombolytic methods. On the one hand, conventional catheter thrombolysis (CDT), where, for example, “recombinant tissue plasminogen activator” (rtPA) is applied directly into the pulmonary arteries via a thombolysis catheter. The angio-jet catheter also allows local injection of the thrombolytic agent by “power pulse spray method”.
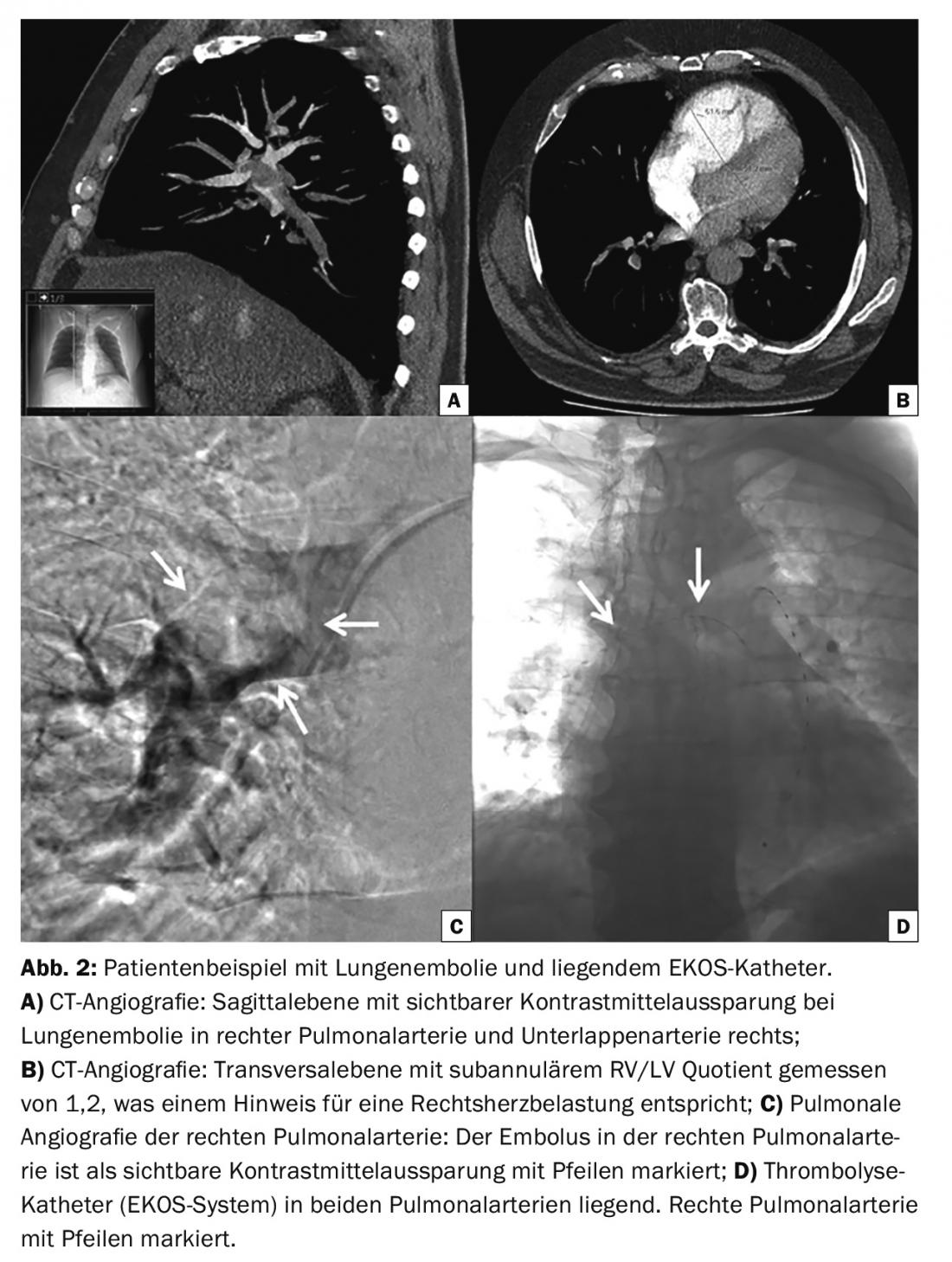
On the other hand, pharmacomechanical thrombolysis using ultrasound-assisted catheter thrombolysis (EKOS system) has been shown to be effective in reducing right ventricular dilatation [11,12]. The ultrasound waves themselves cannot dissolve the thrombus, but they assist fibrinolysis by helping to dissolve fibrin threads and improve penetration of the thrombolytic agent. Figure 2 shows an example of a patient with pulmonary embolism with the EKOS catheter in place.
Literature:
- Kucher N, et al: Massive pulmonary embolism. Circulation 2006; 113(4): 577-582.
- Konstantinides SV, et al: 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) Endorsed by the European Respiratory Society (ERS). Eur Heart J 2014; 35(43): 3033-3073.
- Jimenez D, et al: Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Archives of Internal Medicine 2010; 170(15): 1383-1389.
- Aujesky D, et al: Derivation and validation of a prognostic model for pulmonary embolism. American Journal of Respiratory and Critical Care Medicine 2005; 172(8): 1041-1046.
- Caldeira D, et al: Non-vitamin K antagonist oral anticoagulants and major bleeding-related fatality in patients with atrial fibrillation and venous thromboembolism. A systematic review and meta-analysis. Heart 2015; 101(15): 1204-1211.
- Meneveau N, et al: Management of unsuccessful thrombolysis in acute massive pulmonary embolism. Chest 2006; 129(4): 1043-1050.
- Wan S, et al: Thrombolysis compared with heparin for the initial treatment of pulmonary embolism. A meta-analysis of the randomized controlled trials. Circulation 2004; 110(6): 744-749.
- Meyer G, et al: Fibrinolysis for patients with intermediate-risk pulmonary embolism. The New England Journal of Medicine 2014; 370(15): 1402-1411.
- Jaff MR, et al: Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension. A scientific statement from the American Heart Association. Circulation 2011; 123(16): 1788-1830.
- Engelberger RP, Kucher N: Catheter-based reperfusion treatment of pulmonary embolism. Circulation 2011; 124(19): 2139-2144.
- Kucher N, et al: Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014; 129(4): 479-486.
- Engelberger RP, Kucher N: Ultrasound-assisted thrombolysis for acute pulmonary embolism. A systematic review. Eur Heart J 2014; 35(12): 758-764.
CARDIOVASC 2016; 15(2): 18-22