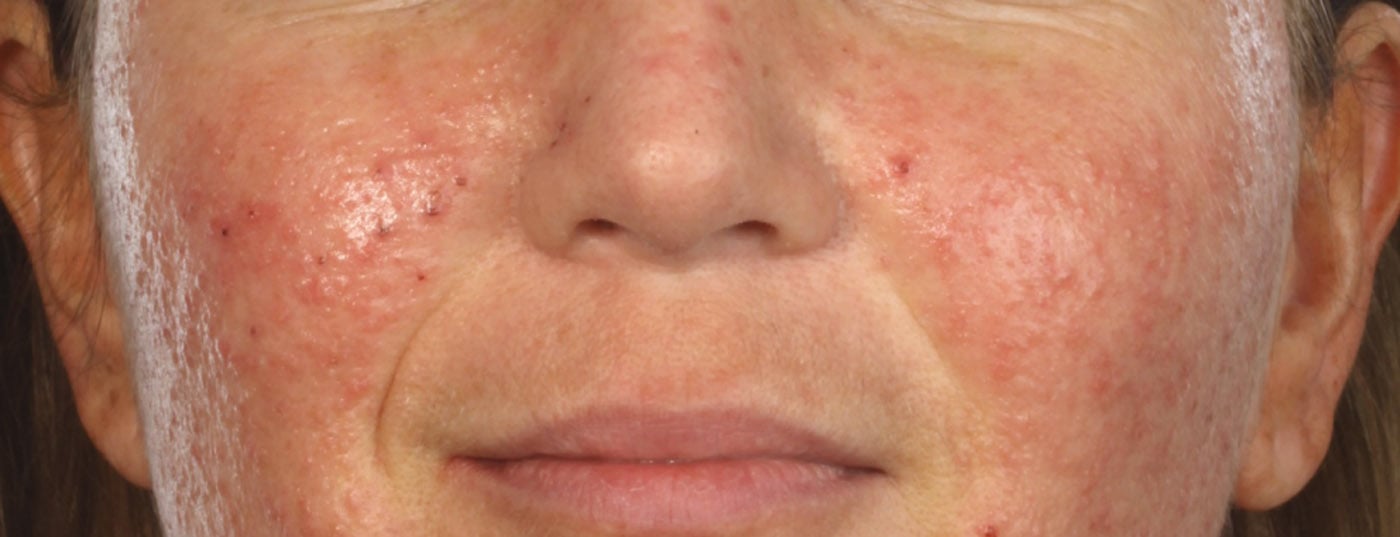
Facial erythema in rosacea is a great burden for patients. On the one hand, it may be accompanied by unpleasant symptoms such as burning, stinging, flushing, tightness, and itching; on the other hand, it is stigmatizing and thus has a psychosocial component. At the Swiss Derma Day in Lucerne, the possibilities of therapy were discussed.
“Rosacea is a common inflammatory skin disease with a prevalence of 5-10% in Europe. It usually begins between the ages of 30 and 50 . Women are affected about three times as often as men,” explained Prof. Dr. med. Bernhard Homey, Director of the Department of Dermatology at the University Hospital Düsseldorf. There are several clinical subtypes (Table 1) .

Possible symptoms of rosacea include “flushing” or persistent erythema, papules, pustules, phymata (e.g., rhinophyma), burning, itching, pain, and ocular involvement. The general therapeutic measures are sufficiently known, but their importance in the overall treatment concept cannot be sufficiently pointed out:
- Avoidance of trigger factors such as UV exposure, heat, cold, food spiciness, stress.
- Use of appropriate cosmetics including soap-free skin cleansing.
- No manipulation.
In the systemic therapy of moderate to severe papulopustular rosacea (subtype II), 16 weeks of treatment with doxycycline at an anti-inflammatory (non-antibiotic) dose of 40 mg/d has been shown to be effective and safe [1]. A treatment effect can be seen after only three weeks. Tolerability is good and side effects are comparable to placebo (no candidiasis or phototoxicity). However, what was not observed with doxycycline was a significant effect on erythema.
What’s new?
Since January 2015, the 0.33 percent brimonidine gel called Mirvaso® (corresponding to 0.5 percent brimonidine tartrate) has now been available on the Swiss market for the first time as an active ingredient that also shows a significant effect against rosacea-related erythema, which is difficult to control. It is an α2-adrenoreceptor agonist with potent vasoconstrictor activity. The gel is applied within 24 hours at any time that is convenient for the patient, as long as the facial erythema persists. Since the effect lasts for about 12 hours, the relevant time for the affected person is covered if applied in the morning. As needed, the patient can control the application individually. It is important that patients do not apply the gel before going to bed and thereby sleep through the effect. Recommended maximum daily dose is 1 g total, divided into five small pea-sized amounts.
In two phase II studies (subtype I rosacea), brimonidine tartrate at the 0.5% concentration was found to be particularly effective [2]. In Study A, with a single application, measurement of skin redness by Chroma Meter showed significant improvement over the drug-free vehicle as early as 30 minutes after application. The maximum effect occurred after 4-6 hours – in total, the effect lasted up to 12 hours. In study B, with once-daily application for four weeks, 0.5% brimonidine tartrate gel was also superior to vehicle: Significantly more patients in the verum group achieved two-degree improvement in erythema according to physician’s and patient’s self-assessments (Clinician’s Erythema Assessment, CEA; Patient’s Self-Assessment, PSA) on days 1, 15, and 29 over the entire 12 hours.
Erythema in rosacea
Prof. Dr. med. Martin Schaller, deputy. medical director of the University Dermatological Clinic in Tübingen, again went into more detail about erythema and the current study situation: “Regardless of whether the vasomotor dysregulation was triggered by neurogenic, hormonal, thermal, topical or other stimuli, its end result is always a persistent abnormal dilation of the blood vessels of the face. Therefore, the topical application of brimonidine to reduce facial erythema is directly based on its pharmacological vasoconstrictor effect.”
The positive results of two phase III trials [3] were pivotal for Swissmedic approval: patients with moderate to severe erythematous rosacea were randomized to receive either 0.5% brimonidine tartrate (n=277) or the drug-free vehicle (n=267). The treatment and follow-up period was four weeks each. Treatment effect was assessed by CEA and PSA (Table 2) .
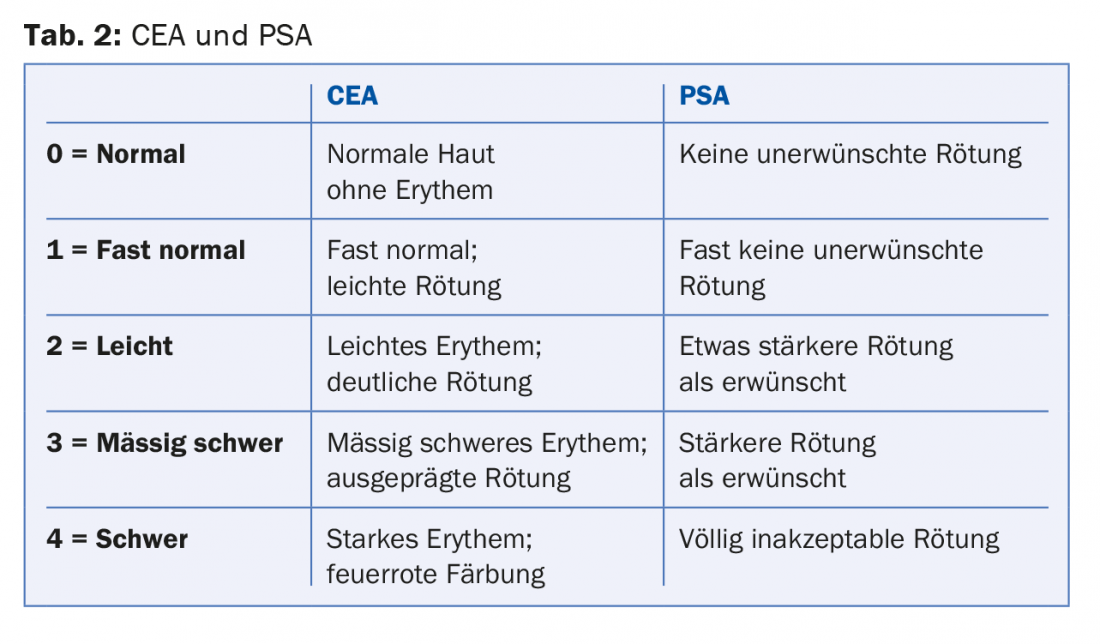
Compared with the vehicle group, significantly more patients in the verum group achieved a clinically relevant improvement of one grade in the CEA and PSA scales over the entire 12 hours on days 1, 15, and 29. After the treatment period, the mean changes in the two scales decreased again, as expected, but there was no rebound phenomenon (the CEA and PSA scores were not above baseline at day 0 even after the four-week follow-up). Neither group showed a clinically relevant response in terms of telangiectasia or inflammatory lesions.
In 2014, Moore and colleagues [4] demonstrated the long-term efficacy and safety of once-daily application over a total period of twelve months in 449 patients. This showed that the incidence of side effects (including those associated with the tested therapy) tended to decrease over the course of the study: While in the first quarter 7.1% of patients discontinued the study due to at least one therapy-related side effect, this was only 2.3% at the end. The most common problems associated with the gel were as follows:
- Enhancement of erythema (6.5%)
- Flushing (9.1%)
- Burning (3.3%)
- Skin irritations (3.1%)
- Rosacea (3.6%).
Overall, there were no serious new safety-related findings or signals during long-term use and no evidence of an increase in risk for any particular type of adverse event. The authors observed no clinically relevant changes in the number of telangiectasias or inflammatory lesions as a result of the gel-but the safety profile was not worsened in those subjects who underwent concurrent therapy for inflammatory lesions.
Rebound still possible?
After US approval, there were case reports of rebound effects. After an initially satisfactory result, increased flushing or erythema was observed within the first days of treatment compared to baseline. These deteriorations initially persisted even after discontinuation of the gel, but then subsided after a few days or weeks, indicating a transient side effect. The published cases were patients with concomitant autoimmune diseases and under comedication. “You can’t call it a true rebound in these cases; rather, it was a symptom exacerbation. Nevertheless, we must of course inform physicians about the possibility of such a problem (tab. 3) and select suitable patients for therapy,” says Prof. Schaller.

Tolerance should be tested on a limited skin area. Patients must also be instructed in the correct use (adhere to recommended dose). Side effects must be reported immediately to the attending physician.
Source: Symposium at Swiss Derma Day, January 28-29, 2015, Lucerne
Literature:
- Del Rosso JQ, et al: Two randomized phase III clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol 2007 May; 56(5): 791-802.
- Fowler J, et al: Once-daily topical brimonidine tartrate gel 0.5% is a novel treatment for moderate to severe facial erythema of rosacea: results of two multicentre, randomized and vehicle-controlled studies. Br J Dermatol 2012; 166(3): 633-641.
- Fowler J, et al: Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol 2013 Jun 1; 12(6): 650-656.
- Moore A, et al: Long-term safety and efficacy of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of a 1-year open-label study. J Drugs Dermatol 2014 Jan; 13(1): 56-61.
DERMATOLOGIE PRAXIS 2015; 25(3): 28-30