Oncotype DX®, MammaPrint® and EndoPredict® are validated gene assay tests with proven prognostic value. The use of the tests can currently be used in early breast carcinoma (Luminal A/B) in the intermediate range for decision support. Prospective randomized studies on the predictive value of the tests (TailorX, PlanB and MINDACT) are still pending. The costs have been covered by the health insurance funds since January 2015.
Which patients benefit from adjuvant chemotherapy and which do not is one of the core questions of any interdisciplinary board. The assays currently on the market promise better specificity compared to conventional clinicopathological parameters and should help avoid unnecessary chemotherapy. Particularly in patients with so-called intermediate risk profiles, there is treatment uncertainty, which the assays are designed to address. Previously, the risk of recurrence was assessed based on risk factors such as tumor biology, grading, tumor spread, lymph node status, and age. As early as 2000, the work of Perou et al. described the heterogeneity of tumors and their classification into molecular subtypes. The formation of gene expression clusters results in the intrinsic subtypes such as luminal (A and B), Her2 enriched and basal like. However, recent data show that these subtypes are not intrinsically uniform and thus also show different prognoses. Based on the determination of hormone receptors, the Her2 receptor and the proliferation factor Ki67, these subtypes, classified as gene expression clusters, can also be detected by immunohistochemistry.
Classification of patients into Luminal A or B is imprecise in the intermediate range of Ki67. As a result, patients with early breast cancer may receive chemotherapy unnecessarily.
What tests are available?
The following tests are currently the most widely used in Europe:
Oncotype DX® (GenomicHealth Inc., Redwood City, USA): Is a prognostic test for estrogen receptor-positive breast cancer patients. The test is performed using reverse transcription followed by quantitative PCR (RT-qPCR). The tissue is formalin-fixed and embedded in kerosene (FFPE). Gene expression levels of 21 genes (16 information genes and five reference genes) are determined (Fig. 1).
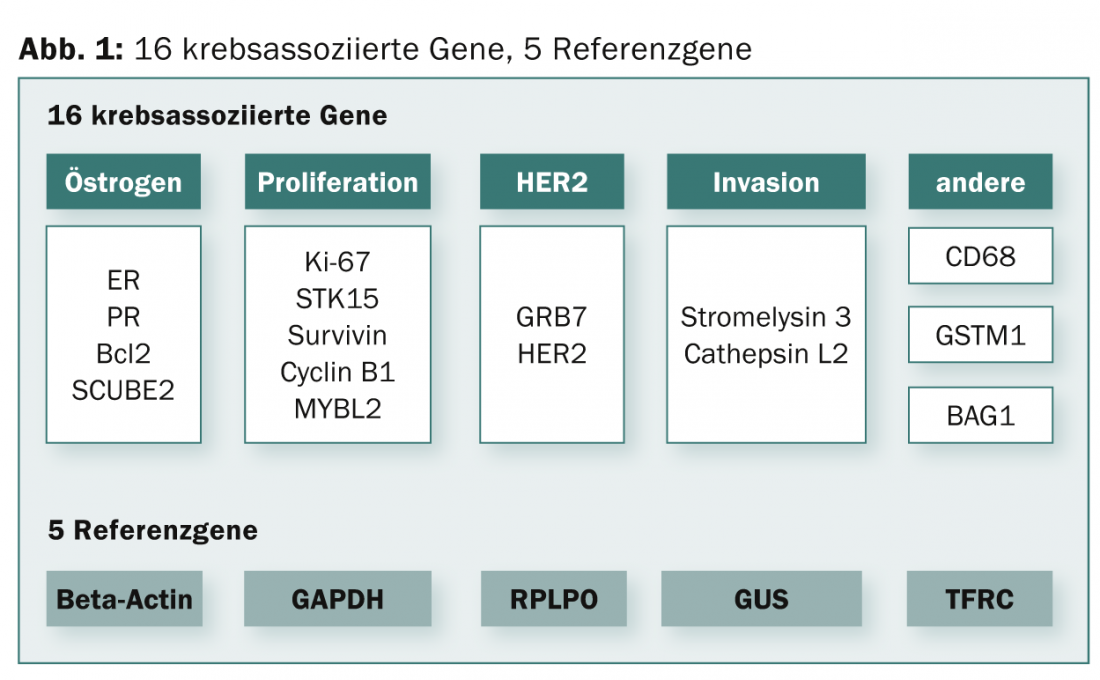
A mathematical algorithm is then used to calculate the recurrence score (RS). The RS is a continuous prognostic score (scale: 1-100), which today is still divided into three risk groups (“low-risk”, RS: <18; “intermediate-risk”, RS: 18-30; “high-risk”, RS: >30). The test is performed centrally in the USA. A large number of prospective-retrospective validation studies are available [1–3]. Health economic analyses/decision impact studies are available [4–6].
The predictive value of the test was retrospectively evaluated for response to chemotherapy with CMF or FAC (5-fluorouracil, doxorubicin/adriamycin, cyclophosphamide) in archieved tumor tissue. Patients with low RS showed no benefit from this chemotherapy [7].
EndoPredict® (Sividon Diagnostics GmbH, D): Is a prognostic test for ER-positive, HER2-negative breast carcinomas. It is determined by RT-qPCR using FFPE. The gene expression level of twelve genes (eight information genes and four reference genes) is determined and a prognostic score (EP) is calculated using a mathematical algorithm. The score ranges from 1 to 15 and has two risk groups: EP ≤5 = “low-risk”, EP >5 = “high-risk”. In addition, the EndoPredict® test was supplemented with a molecular-clinical-pathological risk score (EPclin Score). The nodal status and tumor size are included in the risk classification. This also results in two risk classes in “low-risk”, EPclin ≤3.3 and “high-risk”, EPclin >3.3 (Fig. 2).
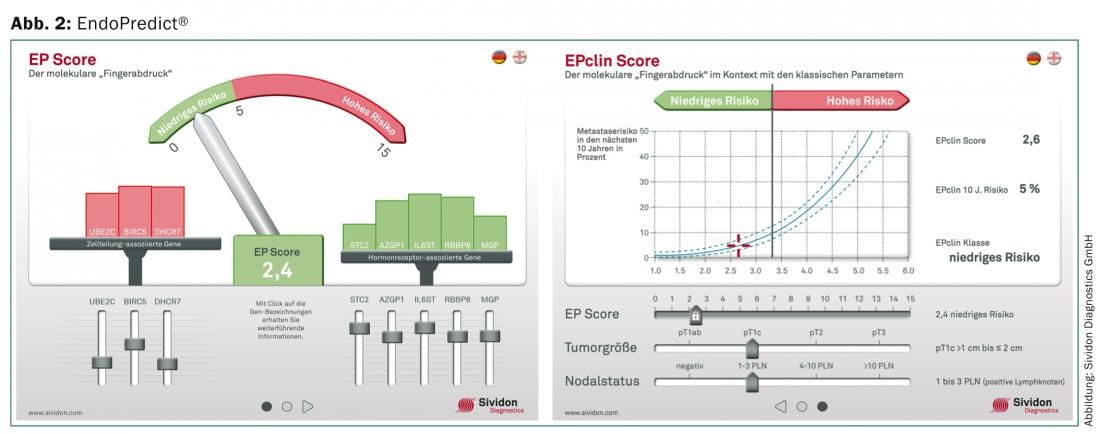
The EndoPredict® can be performed decentrally in molecular pathology. Comparative studies on uPA, PAI-1 are available [8]. So far, the test can only be performed in Germany, Austria and Switzerland. Numerous prospective retrospective validation studies [9] are available, but prospective randomized trials are lacking to date.
MammaPrint® (Agendia, Amsterdam, NDL): Is the first FDA-approved gene assay test. It is a microarray-based gene expression assay that previously could only be performed using fresh tissue. This was a disadvantage in handling. Recently, the test can also be performed with FFPE tissue. A total of 70 information genes and several hundred reference genes are quantified in the tumor tissue in order to perform the risk classification. There are also only two risk groups for this test. The low-risk group (“good prognosis”) shows a significantly better clinical course than the high-risk group (“poor prognosis”). This was shown by numerous independent validation studies [10]. The test is performed in a central laboratory in Amsterdam.
Recommendations from the guidelines
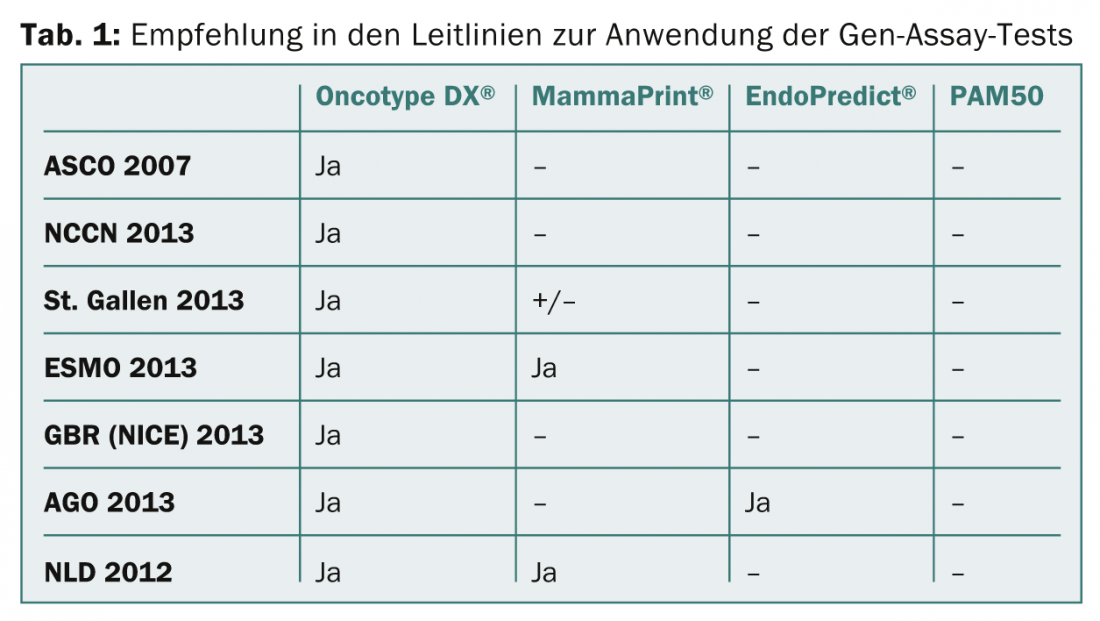
In the AGO and S3 guidelines for the treatment of breast carcinoma, the Oncotype DX® and the EndoPredict®/EPclin test are recommended for use in individual cases. In particular, Oncotype DX® has the widest acceptance in guidelines (ASCO 2007, NCCN2013, St Gallen 2013, ESMO 2013, GBR [NICE] 2013) (Table 1).
Prognostic and predictive factors
Prognostic factors estimate the patient’s disease course. This does not take adjuvant therapy into account and thus only predicts how good or bad the patient’s baseline is. Prognostic are all of the above tests.
Predictive factors are used to assess the effectiveness of a particular adjuvant therapy (treatment response). Predictive factors should help determine which chemotherapy a patient may benefit from.
Summary
Prognosis of early breast carcinoma has been previously determined based on immunohistochemical analysis. The proliferation factor Ki67 is decisive for the classification in subgroups Luminal A or B. Immunohistochemical determination of Ki67 is inaccurate, especially in the intermediate range. This has been shown in interlaboratory tests. Here, gene assay tests promise better specificity. What is essential, however, is whether these tests are also predictive and thus can predict the benefit of chemotherapy. Current data show up to a 45 percent reduction in primary planned chemotherapy regimens when using the gene assays [11,12]. The tests thus have an unmistakable influence on the decision-making of the treating physicians. A prospective randomized multicenter study was completed last year as part of SAKK (SAKK26/10). It is intended to demonstrate the impact of Oncotype DX® on physician decision making regarding adjuvant therapy. The results are expected in 2016.
Two large prospective randomized trials with more than 15 000 patients (TailorX, PlanB) have been completed. First results will be available at the end of 2015 resp. available at the beginning of 2016. Both studies provide data on the predictive utility of Oncotype DX®. TailorX is also designed to provide a breakdown of the intermediate range of the recurrence score. Another prospective randomized study (MINDACT) will provide us with data regarding the predictive value of MammaPrint®.
Until these data are available, retrospective studies will be used to support the justification for use of the assays. Since the beginning of January 2015, the costs are covered by the health insurance companies in Switzerland.
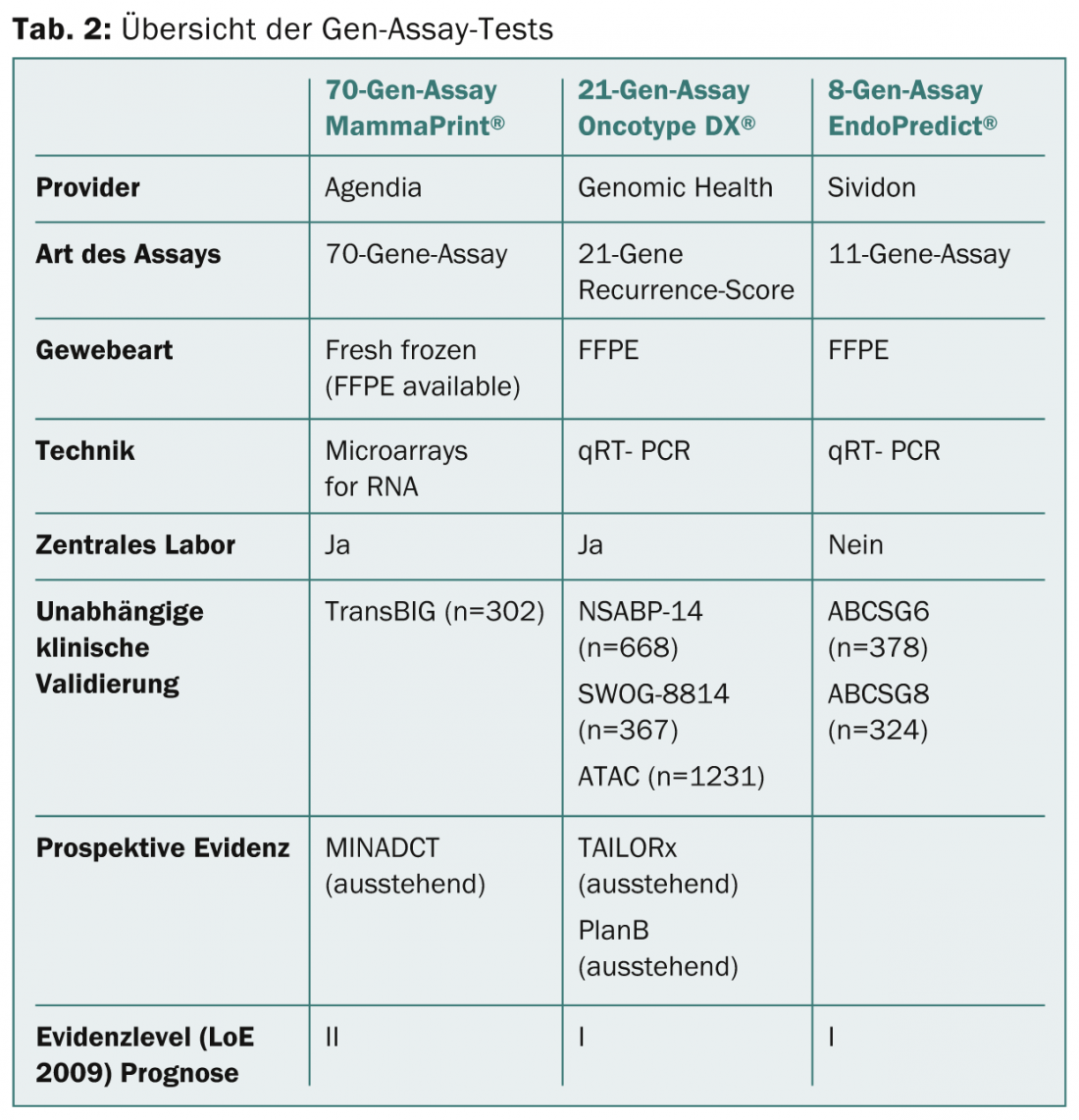
Table 2 summarizes the gene assay tests mentioned above.
Literature:
- Paik S, et al: A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004; 351(27): 2817-2826.
- Albain KS, et al: Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, estrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. The Lancet Oncology 2010; 11(1): 55-65.
- Dowsett M, et al: Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol 2010; 28(11): 1829-1834.
- Klang SH, et al: Economic implications of 21-gene breast cancer risk assay from the perspective of an Israeli-managed health-care organization. Value in health: the journal of the International Society for Pharmacoeconomics and Outcomes Research 2010; 13(4): 381-387.
- Kondo M, et al: Economic evaluation of the 21-gene signature (Oncotype DX) in lymph node-negative/positive, hormone receptor-positive early-stage breast cancer based on Japanese validation study (JBCRG-TR03). Breast Cancer Res Treat 2011; 127(3): 739-749.
- Geffen DB, et al: The impact of the 21-gene recurrence score assay on decision making about adjuvant chemotherapy in early-stage estrogen-receptor-positive breast cancer in an oncology practice with a unified treatment policy. Ann Oncol 2011; 22(11): 2381-2386.
- Paik S, et al: Gene Expression and Benefit of Chemotherapy in Women With Node-Negative, Estrogen Receptor-Positive Breast Cancer. JCO Early Release, published online ahead of print May 23 2006.
- Ettl J, et al: Prospective comparison of uPA/PAI-1 and EndoPredict-clin score in ER-positive, HER2-negative breast cancer: Impact on risk stratification and treatment decisions. J Clin Oncol 2013; 31 (suppl; abstr 581).
- Filipits M, et al: A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin Cancer Res 2011; 17(18): 6012-6020.
- Buyse M, et al: Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J Natl Cancer Inst 2006; 98(17): 1183-1192.
- Goodwin MCHJ, Diego M, Frazier TG: Impact of Oncotype DXTM recurrence score and tumor size on making chemotherapy decisions in breast cancer patients. Paper presented at the San Antonio Breast Cancer Symposium 2009.
- Guth AAFS, et al: Utilization of Oncotype DX to predict chemotherapy use in an innercity population. Paper presented at the American Society of Clinical Oncology Annual Meeting 2011.
InFo ONCOLOGY & HEMATOLOGY 2015; 3(2): 5-8.











