Orthodox medicine makes no distinction between so-called “natural” and “synthetic” vitamins. In studies with vitamins, the origin of the vitamin preparations used is practically never mentioned. However, there are providers who distinguish between synthetic and natural vitamins and point to the few studies that look at differences between the two types of vitamins.
Not only do specialty stores such as pharmacies and drugstores offer numerous vitamin preparations, but health food stores, fitness centers and large-scale distributors also have shelves full of corresponding products. In specialty stores, people are always asking for so-called “natural” vitamins. And indeed, there are pharmaceutical companies that offer natural vitamins.
This raises the following question: Are there differences between these natural and synthetic vitamins in terms of
- the chemical structure?
- the absorption/bioavailability?
- the effectiveness?
- adverse effects?
This question is of little concern to orthodox medicine, and studies practically never distinguish whether the preparations used are natural or synthetic vitamins.
Definition
It is natural to assume that natural vitamins are obtained from nature by extraction from plants and synthetic vitamins are “chemically” produced. However, this is not the correct definition, because surprisingly, natural vitamins do not necessarily have to be extracted from nature.
A vitamin may be called natural or nature-identical if it chemically corresponds to the form occurring in nature and is present with its natural accompanying substances. Proponents of natural vitamins emphasize that it is these natural adjuncts that can have an impact on absorption and bioavailability. Furthermore, preparations with natural vitamins must not contain any absorbable foreign substances such as synthetic preservatives, colorants or sweeteners.
According to the structure, synthetic and natural vitamins do not differ, so their structural formula is identical. However, there are differences in stereoisomerism.
Vitamin A
The discussion of synthetic and natural vitamin A centers on beta-carotene, as a precursor (also called provitamin A), and the various retinoids called vitamin A. Natural beta-carotene (better carotenoids) is a mixture of cis-beta-carotene, all-trans-beta-carotene, alpha-carotene, etc. (Fig. 1). This carotenoid spectrum is very similar to that of fruits and vegetables.
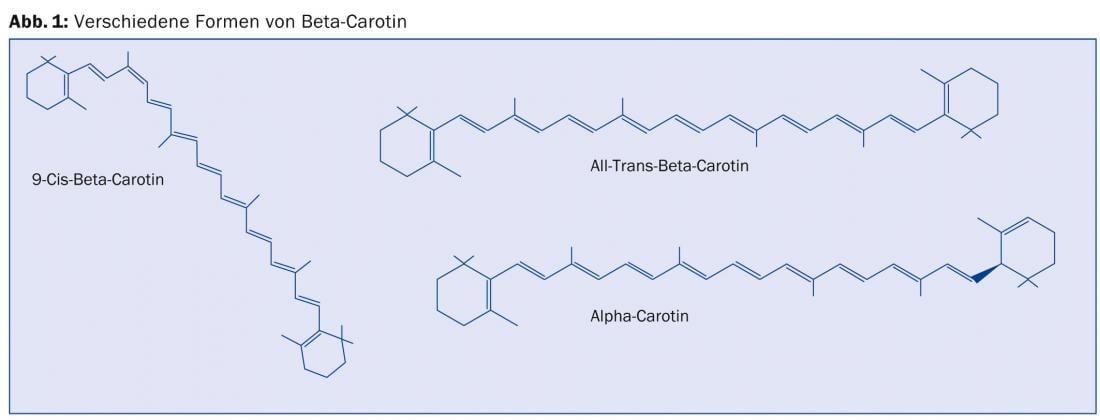
In contrast, the laboratory synthesis of beta-carotene produces only all-trans beta-carotene. According to Challem [1] and Xue et al. [2], there are stereoisomeric differences in efficacy and adverse effects that are to the disadvantage of the synthetic variant. According to Challem, natural carotenoid mixtures are antioxidants with health-maintaining properties. Synthetic carotenoid also shows benefit, but also has negative effects, as retinol formed in the body from synthetic beta-carotene increases the risk of liver disease and precancerous cell changes in association with high alcohol intake.
Vitamin B
All eight compounds of the vitamin B complex exist in nature exactly as they are formed in laboratory synthesis. So there are no stereoisomeric differences either. Therefore, even suppliers of natural vitamins resort to laboratory synthesis for vitamin B. Only in the case of folic acid, formerly sometimes called vitamin B9 or B11, is there an advantage to absorption for the natural form because natural folic acid is in a plant matrix that increases the absorption and thus the bioavailability of folic acid.
Vitamin C
There are no chemical differences between ascorbic acid synthesized in the laboratory and that found in nature, not even stereoisomeric differences. However, while synthetic vitamin preparations consist of 100% ascorbic acid, natural ascorbic acid, like folic acid, is also present embedded in a plant matrix (bioflavonoids, trace elements). As a result, ascorbic acid lingers longer in the gastrointestinal tract, which has a positive effect on absorption and bioavailability. The same effect is achieved with a synthetic ascorbic acid preparation that is available in a sustained-release form.
Vitamin D
With vitamin D, a distinction is made between vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol).
Vitamin D2 has practically no significance and is used almost only by vegans, as it is obtained from lichens and mushrooms.
Today, vitamin D3 is obtained from lanolin/wool fat by treating it with UV rays. By the way, the 7-dehydrocholesterol present in our skin is also converted into cholecalciferol with UV light. Comparative studies each show significantly better bioavailability of vitamin D3 compared to vitamin D2.
Interestingly, vitamin D is never synthesized in the laboratory from starting materials. So you can’t distinguish between a natural and a synthetic form here.
Vitamin E
However, there are real differences when it comes to vitamin E. The difference between synthetically produced and naturally occurring alpha-tocopherol, called vitamin E, is caused by chirality. The naturally occurring alpha-tocopherol is exclusively dextrorotatory and is therefore referred to as d-alpha-tocopherol. A laboratory synthesis of alpha-tocopherol produces a mixture of l- and d-alpha-tocopherol, i.e. a racemate. The d-alpha-tocopherol found in vegetable oils is present there alongside d-beta-, d-gamma- and d-delta-tocopherol. However, since our body needs only the d-alpha-tocopherol, the other forms are eliminated during the extraction of the natural vitamin E preparations.
The differences between natural and synthetic alpha-tocopherol include the following:
- The bioavailability of d-alpha-tocopherol is significantly better than that of the racemate [3].
- Preferential enrichment of VLDL, LDL and HDL as well as erythrocytes and blood plasma with the natural form [4].
- Preferential accumulation of natural vitamin E in tissues [5].
These differences, of course, document a benefit to the natural form of vitamin E.
Summary
In the case of vitamins B and D, no chemically relevant differences are known today between the forms occurring in nature and those synthesized in the laboratory. Folic acid, which is part of the vitamin B complex, is naturally present in a plant matrix that improves absorption compared to the synthetic form. The same is the case with vitamin C.
In vitamin A or its precursor, beta-carotene, there are stereoisomeric differences between the natural and synthetic forms, with reported advantages for the natural form in terms of pharmacokinetics, efficacy, and adverse effects. In the case of vitamin E, the natural form is a dextrorotatory compound, while synthesis results in a racemate. Studies document benefits for natural d-tocopherol.
These facts speak more in favor of natural vitamins, especially in terms of absorption and bioavailability. So, obviously, it pays to use natural vitamins when taking a vitamin supplement.
Literature:
- Challem JJ: Beta-Carotene and Other Carotenoids: Promises, Failures, and a New Vision. Orthomolec Med 1997; 12: 11-19.
- Xue KX, et al: Comparative studies on genotoxicity and antigenotoxicity of natural and synthetic beta-carotene stereoisomers. Mutation Research 1998; 418(2-3): 73-78.
- Acuf RV, et al: Relative bioavailability or RRR- and all-rac-alphaTocopheriyl in humans. Am J Clin Nutr 1994; 60: 397-402.
- Traber MG, et al: RRR- and SRR-alpha-tocopherols are secreted without discriminationin human chylomicrons, but RRR-alpha-tocopherol is preferentially secreted in very low densitiy lipoproteins. J Lipid Res 1990; 31(4): 675-685.
- Burton GW: Human plasma and tissue alpha-tocopherol concentrations in response to supplementation with deuterated natural and synthetic vitamin E. Am J Clin Nutr 1998; 67(4): 669-684.
HAUSARZT PRAXIS 2014; 9(12): 4-5