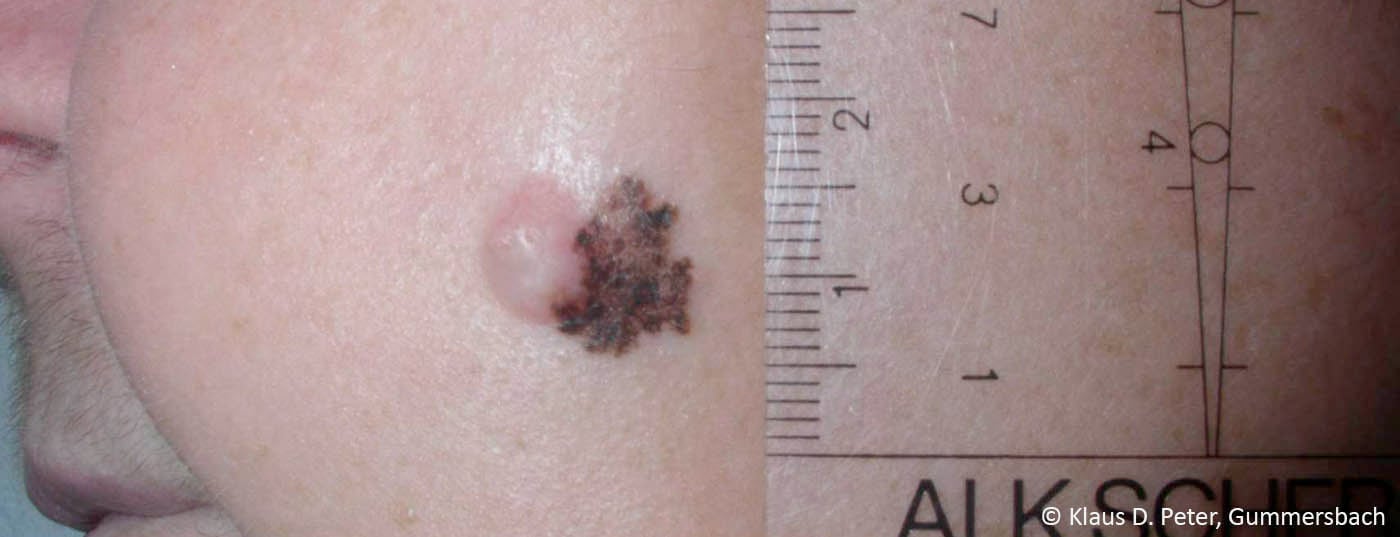
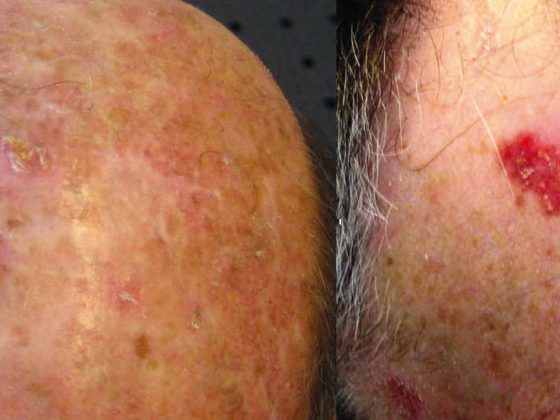
New insights in tumor biology and immunology as well as in molecular pathology have rapidly driven the development of immunomodulatory antibodies and so-called “targeted” therapeutics [1]. This makes the therapy of advanced melanoma an interdisciplinary challenge. Thus, modern treatment of advanced melanoma is increasingly personalized and also based on the mutation status of the tumor.
Surgical resection remains a valid option for solitary organ metastases and, like burdensome limb perfusion with cytostatics and interferon-α, can provide long-term relapse-free survival. Various chemotherapies, especially for melanoma without a mutation to target therapeutically, include, for example, dacarbacin, vinblastine, cisplatin, taxol, and bleomycin, and occasionally tamoxifen as hormonal therapy. Moderate response rates are at the expense of toxicity without significantly affecting overall survival. Temozolomide, an alkylane with a comparable response rate to dacarbazine, can be used frequently for brain metastases because of its cerebrospinal fluid (CSF) penetrability, although fewer than 10% of patients have an objectifiable response to this therapy.
Immunotherapies
Due to the antigenic and immunogenic nature of malignant melanoma, various therapeutic attempts with immunomodulatory agents have been made for a long time. In addition to peptide vaccines (often with CpG oligonucleotide adjuvant), cytokines (e.g. recombinant IL-2) are being tested and immunotherapy methods such as adoptive T cell transfer with TILs (tumor infiltrating lymphocytes) and peripheral blood lymphocytes (PBLs) or genetically modified “engineered” T cells (“chimeric antigen receptor” and TCR) are being developed. High-dose IL-2 (and combination IL-2 with gp100) resulted in long-lasting remissions in a small number of patients, but is predominantly established in the US [2]. Currently, several studies on adoptive T cell transfer and TCR-modified cells as well as checkpoint blockades are ongoing.
CTLA-4 antibody: The method of checkpoint blockade (e.g., CTLA-4 blockade) has successfully enabled new targets for immunomodulatory therapy. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) competes with CD28 for binding to antigen-presenting cells, leading to inhibition of costimulatory signals for T cell activation. However, when CTLA-4 is blocked by a specific human IgG1 monoclonal antibody such as ipilimumab, a “brake” of endogenous defense cells is released and the T cell-mediated tumor response is enhanced. Ipilimumab (Yervoy®), as the first antibody directed against CTLA-4, has also shown significantly prolonged survival in combination with a gp100 peptide vaccine (median survival 10.1 vs. 6.4 monte of vaccine control) and has been approved by the EMA for the treatment of metastatic melanoma since 2011 [3]. Likewise, the combination of dacarbacin with CTLA-4 blockade resulted in durable, complete remissions (3-year survival rate approximately 20%) and raises the prospect of long-term survival for the first time despite a low response rate (approximately 15%). Because a response to ipilimumab may not occur until 12 weeks after initiation of therapy and the drug sometimes induces severe immune-mediated side effects, it is a disadvantage that no prognostic marker has yet allowed patient selection. Therefore, early diagnosis and prompt adverse event management of common autoimmune-induced enterocolitis, hepatitis, thyroiditis, pituitary inflammation, neuritis, and dermatitis is important (Table 1).
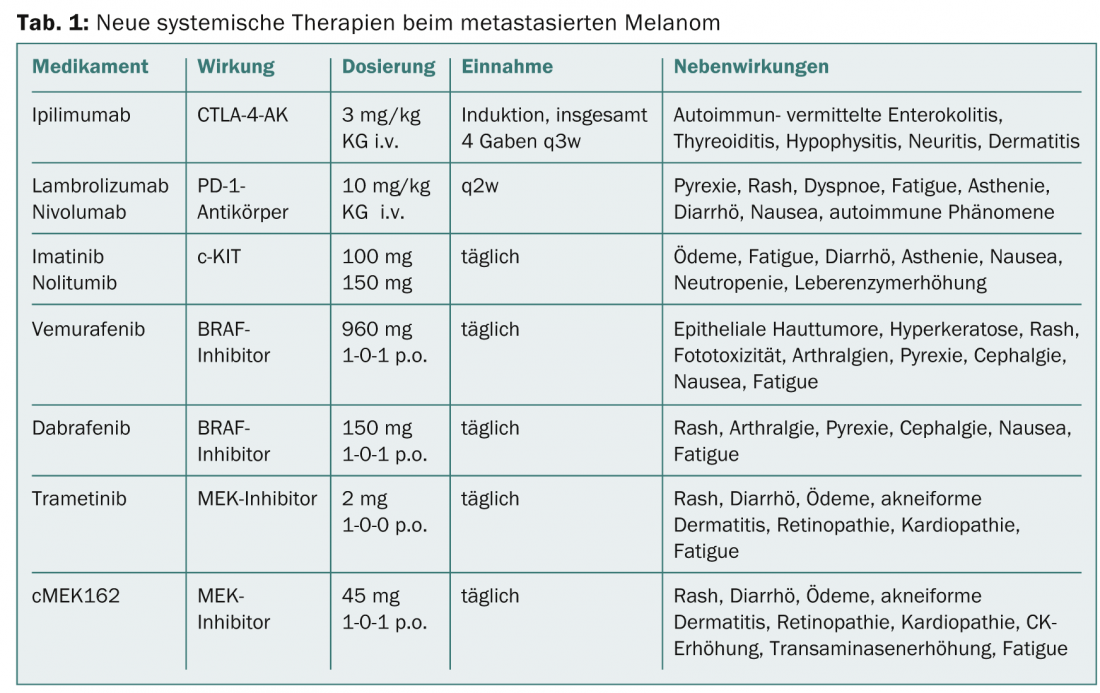
PD-1 and PD-L1 antibodies (PD-1 and PD-L1-AK): With the aim of further optimizing the endogenous immune response against tumor cells, antibodies have recently been developed that inhibit the inhibitory receptor “Programmed Death 1” (PD-1) on the surface of activated T cells. Indeed, if the binding of PD ligand-1 to the PD-1 receptor of the T cell inhibits T cell proliferation, the anti-PD-1 antibodies lambrolizumab and nivolumab, which have been in trials since 2013, can reverse this restriction of the “effector phase”. Initial study results with nivolumab showed a remarkable response in 31% of pretreated melanoma patients [4]. Similar to CTLA-4 blockade, anti-PD-1 antibodies also induce stable remissions with, however, significantly fewer autoimmune-related side effects. In addition, response is usually seen a few weeks after initiation of therapy, with PD-1 ligand expression at the tumor cell surface thought to be a possible predictive biomarker. Lambrolizumab as well as direct antibodies against the PD-1 ligand (PD-L1-AK) are currently in clinical testing. Simultaneous use of CTLA-4- and PD-1-blocking antibodies also shows synergistic effects.
Targeted therapy based on mutation status
Molecular methods and mutational analyses have yielded several interfaces for innovative treatment of metastatic melanoma in the last decade. The most common mutation (approx. 50%) in melanoma cells is a point mutation in the BRAF kinase-encoding gene. BRAF is an important kinase in the MAPK (“Mitogen-Activated Protein Kinase”) signaling pathway and is partly responsible for proliferation and growth of tumor cells. Activating mutations of the BRAF gene V600 lead to overactivation of this signaling pathway resulting in excessive cell growth. Mutations of NRAS, a kinase located downstream of BRAF, are also present in up to 20% of melanoma patients and, like BRAF, cause a worsening of prognosis. Another, rather rare mutation with therapeutic consequence concerns the tyrosine kinase c-KIT, which occurs predominantly in mucosal and acral melanomas. Patients with a c-KIT mutation in exon 11 or 13 respond best to treatment with a c-KIT kinase inhibitor such as imatinib or nilotinib (TEAM trial). Common side effects of therapy include edema, fatigue, diarrhea, loss of appetite, nausea, neutropenia, and liver enzyme elevations.
BRAF inhibitors
The so-called V600 BRAF mutation was also identified as therapeutically predictive in 40-50% of tumors. The first clinically tested BRAF inhibitor, vemurafenib (Zelboraf®), resulted in high response rates in therapy-naïve patients with proven V600E mutation, with 5.6 complete resp. 51.3% partial remissions and to a median overall survival of 13.2 months (Update BRIM-3 trial) [5]. Clinical response usually presents as rapid tumor reduction, although months later resistance often develops due to reactivation of the MAPK pathway or upregulation of alternative pathways.
Another BRAF inhibitor is dabrafenib (Tafinlar®), which has now been approved (BREAK-3 trial) for first-line treatment in non-resectable or metastatic BRAF V600E-positive melanoma [6,7]. The fact that an effective tumor response under dabrafenib is observed in a large proportion of affected patients for an average of eight months improves both quality of life and significantly the expected survival time and represents a new experience in the treatment of advanced melanoma. It was also shown in the BREAK-MB study that patients with brain metastases experience at least a doubling of the otherwise very short survival time by treatment with dabrafenib. Risks from BRAF inhibitor use sometimes include the recurrence of skin tumors, and patients should be monitored regularly for such tumors. Other side effects such as skin and mucosal toxicities, infections, ECG and electrolyte changes, and blood glucose changes may necessitate interruption of therapy. Caution is also indicated in combination with drugs that affect CYP3A4 or CYP2C8 enzyme activity and gastric pH, and concomitant administration of warfarin or digoxin.
MEK inhibitors
As one of the key switch sites in the MAPK signaling chain, and located downstream of RAS-RAF, MEK kinase (MEK1/2 isoforms) can be activated either by mutations or by autocrine growth factors. The highly selective MEK1/2 inhibitor trametinib has been shown to significantly improve progression-free survival (PFS: 4.8 vs. 1.5 months) and overall survival in patients with a BRAF V600E or V600K mutation compared with chemotherapy (METRIC trial) [8]. To date, no development of squamous cell carcinoma has been observed with trametinib, as is common with BRAF inhibitors. In another study, trametinib also showed an effective effect on melanomas with rare BRAF mutations [9]. Another potent and selective MEK1/2 inhibitor, cMEK162, is currently in a phase II trial. Initial results of cMEK162 in untreated melanoma patients with BRAF- or NRAS-mutated melanoma showed comparably good results for both groups (PFS 3.7 vs. 3.6 months), so that for the first time a therapeutic approach also seems possible for patients whose tumors have an NRAS mutation [10].
Combination of BRAF and MEK inhibitors
Due to the observed development of resistance, the combination of a BRAF inhibitor with a MEK inhibitor has now also been tested in a phase I/II trial. After initial dose-finding study, patients with a V600 mutation were administered dabrafenib 150 mg 2× daily. plus trametinib 1 mg or 2 mg daily. or, for comparison, dabrafenib as monotherapy (150 mg 2× daily) [11]. The combination with the higher trametinib dose showed a significantly prolonged PFS by 3.6 months compared to monotherapy with dabrafenib, with 41% of patients in the combination arm still progression-free after 12 months. The most frequent side effects (71%) were pyrexia (>38.5°) and chills under the high-dose combination (Table 1) . These side effects occurred approximately 66% less frequently with dabrafenib monotherapy. As hoped, fewer secondary skin tumors also formed under combination therapy.
Conclusion for practice
- With the approval of ipilimumab (Yervoy®) and vemurafenib (Zelboraf®), immunological and molecular research was successfully translated into the clinic.
- Ipilimumab often leads to long-lasting remissions with a significantly lower response rate.
- Promisingly in the field of immunomodulatory substances, anti-PD-1 antibodies as well as combinations of anti-CTLA-4 and anti-PD-1 antibodies are being clinically tested.
- Despite rapid onset of action and high response rates, BRAF inhibitors often develop resistance, against which combinations with, for example, MEK inhibitors could be successfully used in the future.
PD Andreas Trojan, MD
Literature:
- Dummer R, et al: Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up; ESMO Guidelines Working Group. Ann Oncol 2012 Oct; 23 Suppl 7.
- Smith FO, et al: Treatment of metastatic melanoma using interleukin-2 alone or in conjunction with vaccines. Clin Cancer Res 2008; 14(17): 5610-5618.
- Hodi FS, et al: Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363(8): 711-723.
- Topalian SL, et al: Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014 Apr 1; 32(10): 1020-1030.
- McArthur GA, et al: Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol 2014 Mar; 15(3): 323-332.
- Ascierto PA, et al: Phase II trial (BREAK-2) of the BRAF inhibitor dabrafenib (GSK2118436) in patients with metastatic melanoma. J Clin Oncol 2013 Sep 10; 31(26): 3205-3211.
- Hauschild A, et al: Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012 Jul 28; 380(9839): 358-365.
- Flaherty KT, et al: Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 2012; 367(2): 107-114.
- Kim KB, et al: Phase II study of the MEK1/MEK2 inhibitor trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J Clin Oncol 2013; 31(4): 482-489.
- Ascierto PA, et al: MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol 2013; 14(3): 249-256.
- Flaherty KT, et al: Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 2012; 367(18): 1694-1703.
InFo ONCOLOGY & HEMATOLOGY 2014; 2(9): 19-21.