The goal of surgery for pancreatic cancer is to remove the tumor in healthy tissue with the best possible safety distance of the tumor margin from the resection surfaces. Resectable tumors have the longest life expectancy. Borderline resectable pancreatic cancers are defined according to the extent of infiltration of adjacent organs. In the new S3 guideline, the incision margin in the histopathological assessment is described in more detail than before. An unfavorable prognosis must be inferred if the ratio of affected to tumor-free lymph nodes (lymph node ratio) is ≥0.2. Neoadjuvant therapies seem to be a very promising option.
The 5-year survival rate of pancreatic cancer remains the lowest of all malignancies worldwide at 8%. However, surgical treatment is still of central importance as it is the only potentially curative procedure [1]. Pre-field diagnostics are comprehensive and include the following examinations in addition to basic surveys:
- Routine laboratory
- Tumor markers CA 19-9 and CEA
- HbA1c
- Functional diagnostics with stool elastase and oral glucose tensor test
- X-ray thorax
- Sonography
- Contrast enhanced thin film
- CT Abdomen
- Magnetic resonance cholangiopancreaticography (MRCP)
- Esophago-gastro-duodenoscopy
- Endosonography
- Endoscopic retrograde cholangiopancreaticography (ERCP) with attempted specimen retrieval or stent placement in severe jaundice.
In selected cases, staging laparoscopy should be discussed: When imaging shows no definite findings regarding metastasis to the peritoneum and/or liver, but metastasis is likely because of a high CA 19-9 value of >1000 U/ml, or when prior endosonographic attempts to biopsy for malignancy were unsuccessful [2].
Neoadjuvant therapy may allow for the likelihood of resection depending on the response grade and may allow for subsequent resection of the tumor. Tumors that are not reasonably resectable should be treated with chemotherapy via a previously implanted i.v. port in palliative intent according to the current S3 guideline.
Principles of the operation
The aim of surgery is to remove the tumor in healthy tissue with the best possible safety distance of the tumor margin to the resection surfaces (R0 situation) [3,4]. The standard procedure for a resectable pancreatic head tumor (including processus uncinatus) is partial pancreaticoduodenectomy as a so-called classic or pylorus-preserving Whipple operation with appropriate lymph node dissection (Fig. 1) [5].
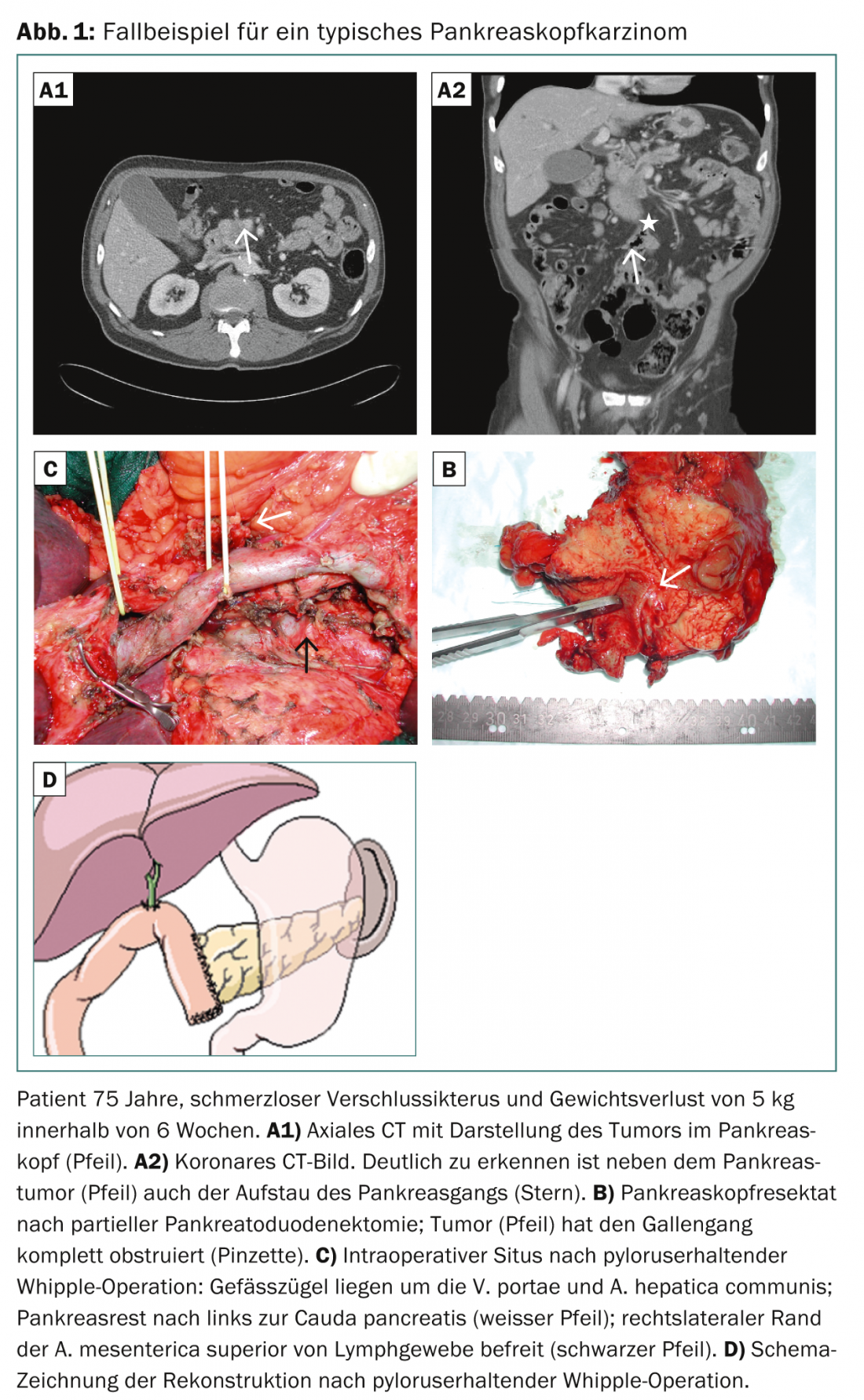
The dissection level of lymph nodes includes peripancreatic and periduodenal lymph nodes, lymph nodes located on the right side of the inferior mesenteric artery, and lymph nodes in the hepatoduodenal ligament. Radical extended lymphadenectomy (interaortocaval, left superior mesenteric artery) does not improve the prognosis and is rather contraindicated due to the additional high postoperative morbidity with chyle fistula and massive diarrhea [6]. If the tumor is located on the left side of the superior mesenteric vein (pancreatic corpus or tail), left resection is performed with splenectomy and appropriate local lymph node dissection.
If it becomes apparent only intraoperatively that distant metastases exist (peritoneum/liver) or that the tumor is not reasonably resectable locally, palliative surgery should be performed. If histological confirmation has not yet been possible, the tumor is biopsied to confirm the diagnosis in such a way that there is only a low probability of morbidity with regard to a pancreatic fistula (e.g., transduodenal or through the bile duct). Clear carcinoma protection is a sine qua non for subsequent palliative or neoadjuvant chemotherapy. Resections of liver metastases are usually not useful.
In cases of cholestasis, a biliodigestive anastomosis is created and the biliary stent is removed intraoperatively. In this situation, it is usually advisable to additionally create a transmesocolic gastroenterostomy even without existing duodenal stenosis. The combination of both procedures is double bypass reconstruction.
Surgical resectability determines prognosis
Despite the high quality of modern imaging techniques, the resectability of a pancreatic tumor can only be assessed by the visceral surgeon. Here, experience of the surgeon and the center play the central role. Patients should therefore present to centers with high case numbers so that locoregional resectability can be assessed. Resectable tumors have the longest life expectancy. One can now achieve 5-year survival rates after oncologic resection with adjuvant chemotherapy of 24-36%. Infiltration of adjacent organs does not necessarily preclude R0 resection. The extended (en bloc) resection required for this purpose promises a comparable prognosis to standard surgery and should be performed in pancreatic centers [7].
The NCCN (National Comprehensive Cancer Network) defines borderline resectable pancreatic carcinomas according to the extent of infiltration of the neighboring organs (Tab. 1) . A “walling” of the superior mesenteric artery or the coeliac trunk of more than 180° is not considered to be reasonably resectable, but to date, according to expert consensus, is not an absolute contraindication for resection. However, one must be aware that perioperative morbidity and lethality rates are increased [8]. In contrast, infiltration of the portal vein, superior mesenteric vein, and lienal vein are not absolute contraindications. En bloc resections of pancreatic and portal venous segments have a comparable complication rate to operations without portal venous vascular infiltration [3,9]. Long-term data from some studies show comparable prognosis to procedures without partial vein resections. In contrast, portal vein infiltration above 2 cm is considered critical and is a negative prognostic factor [10].

Indications for bile duct stent insertion
Tumors of the pancreatic head are usually conspicuous by painless icterus and prompt ERCP. However, insertion of an efferent bile duct stent very frequently leads to bacterial colonization of the bile (approximately 73%) and consecutive cholangitis, which further increases morbidity during pancreatic resections [11]. Therefore, the indication for stent placement should be made in consultation with visceral surgeons and should be strict. However, if surgery cannot be performed promptly for rapidly increasing icterus (above ten times normal), the indication for stenting is essential (good clinical practice criteria, GCP).
Standardized histopathological workup
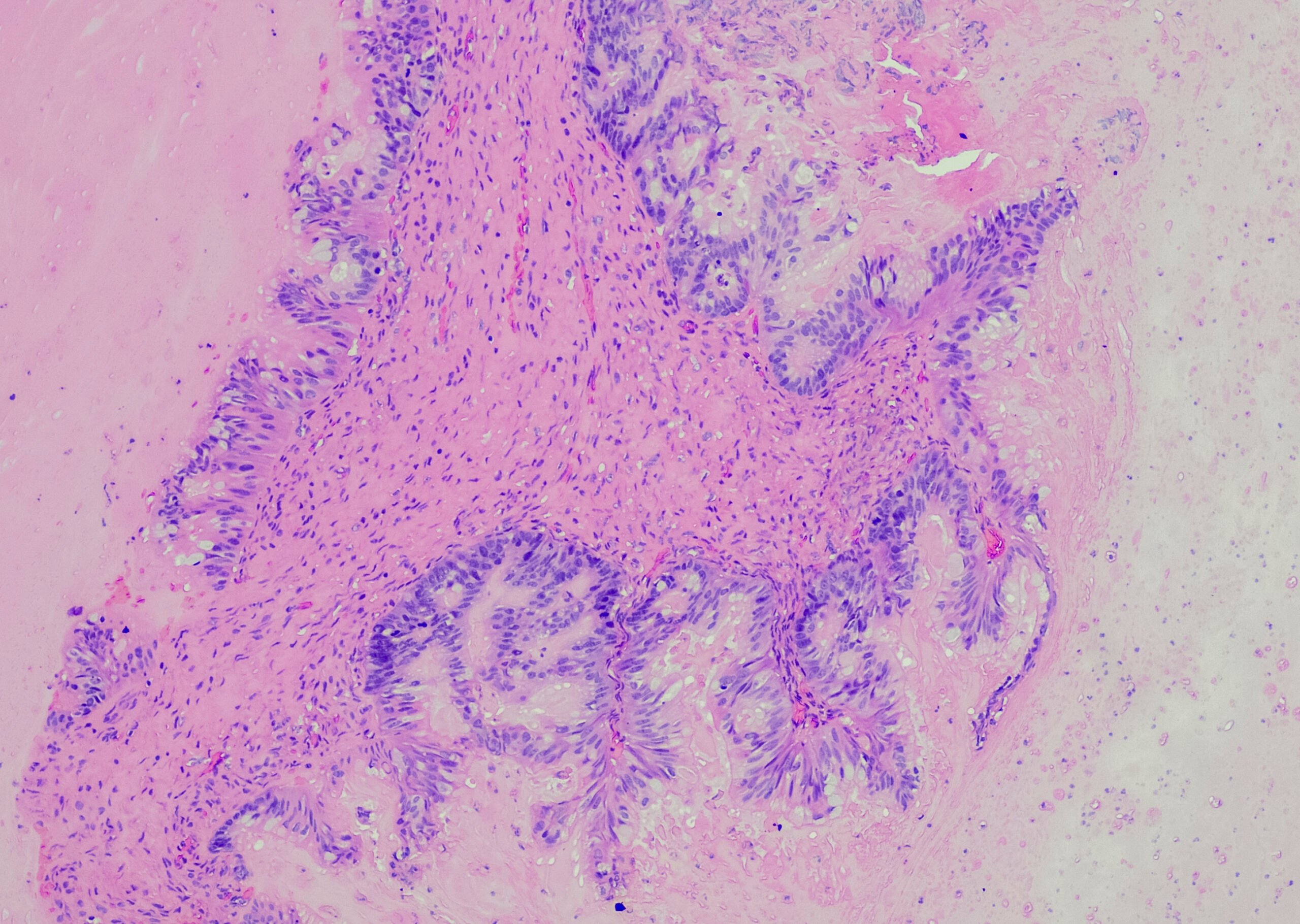
The minimum distance to the incision borders has been discussed controversially in the recent past – this after “arbitrary” redefinition of the R-classification by the “Royal College of Pathologists” (RCP). The RCP already postulates an R1 situation if tumor cells <1 mm to the resection margin are present, but no tumor cells are detectable at the incision site. In the new S3 guideline, it was therefore decided to describe the circumferential incision margin (ventral, medial, and posterior) in more detail in the histopathologic evaluation (CRM concept based on the histologic evaluation of colorectal carcinomas):
- R0 resections are described as CRM-negative (R0-wide) when tumor cells are >1 mm from the resection margin.
- R0 resections are described as CRM-positive (R0-narrow) if the distance of malignant cells to the cut surface is <1 mm (Fig. 2).
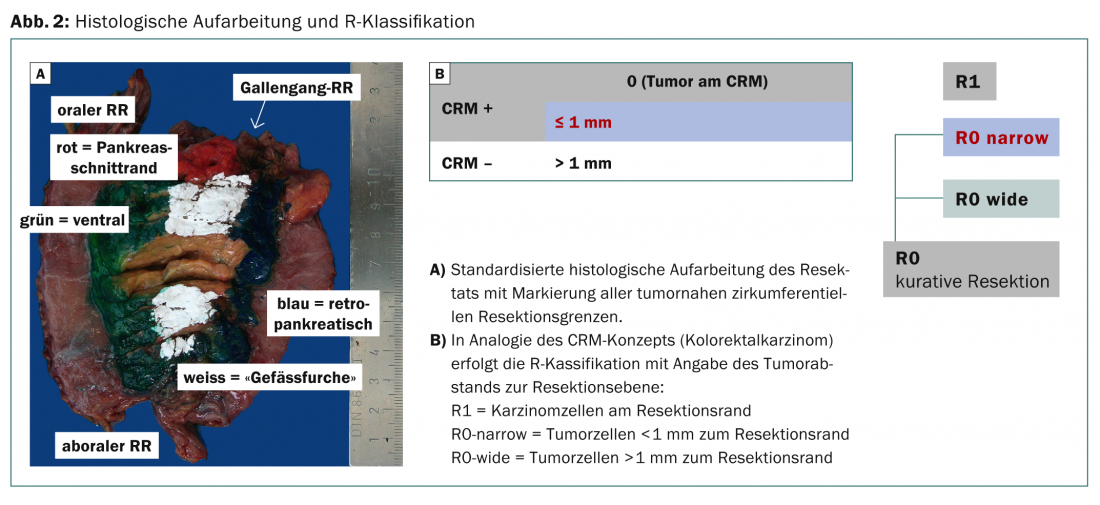
Recent studies show that the absence of tumor cells at the incision margin is the decisive criterion (corresponds to the previous R-definition according to UICC) [12]. The goal of the standardized new histologic workup is to provide better data for estimating prognosis and recurrence probability in the future.
Lymph node ratio is a prognostic factor
The new S3 guideline on pancreatic cancer calls for lymphadenectomy of at least ten locoregional lymph nodes in addition to standardized histologic workup. These are processed in such a way that it is possible to specify the lymph node ratio, i.e. the ratio of affected to non-affected lymph nodes (GCP recommendation). An unfavorable prognosis must be inferred if the ratio of affected to tumor-free lymph nodes is ≥ 0.2 [13].
No resection for distant metastases
If distant metastases are already detected preoperatively, surgical treatment is usually not advisable. These include organ metastases, for example in the liver and lungs, peritoneal carcinomatosis with ascites, or lymph node metastases outside the locoregional areas (considered distant metastases). If previously unknown distant metastases are discovered intraoperatively, resection leads to increased perioperative morbidity and is without survival benefit. However, there is limited literature data on this [14].
Neoaduvant therapy in pancreatic cancer.
Although the trial data on neoadjuvant therapeutic approaches in patients with resectable or borderline resectable tumors (chemoradiotherapy or chemotherapy followed by radiotherapy) are insufficient to date, neoadjuvant therapies appear to be a very promising option [15,16]. In individual unicenter studies, secondary resectability could thus be achieved and the prognosis of the affected patients improved. In particular, better-performing intensified chemotherapy regimens are now available (FOLFIRINOX and gemcitabine/abraxane), allowing significant tumor regression for the first time.
Although no controlled randomized trials have been published to date, neoadjuvant therapy approaches may be gaining acceptance [17]. Thus, patients are currently being recruited into five different trials that will evaluate the value of neoadjuvant treatment even in patients classified as resectable and borderline resectable or borderline resectable. Investigate locally advanced pancreatic tumors. The goal is to increase curative intent R0 tumor resection to improve long-term survival of patients with pancreatic cancer.
Literature:
- Seufferlein T, et al: Dtsch Arztebl Int 2014; 111: 396-402.
- Doi R, et al: Surg Today 2008; 38: 1021-1028.
- Wagner M, et al: Br J Surg 2004; 91: 586-594.
- Hartwig W, et al: Ann Surg 2011; 254: 311-319.
- Chromik AM, et al: Oncologist 2012; 18: 269-282.
- Kahlert C, et al: Surgeon 2008; 79: 1115-1122.
- Nikfarjam M, et al: J Gastrointest Surg 2009; 13: 915-921.
- Yamada S, et al: Pancreas 2009; 38: e13-17.
- Bachellier P, et al: Am J Surg 2001; 182: 120-129.
- Ouaissi M, et al: World J Surg 2010; 34: 2648-2661.
- van der Gaag NA, et al: N Engl J Med 2010; 362: 129-137.
- Janot MS, et al: Langenbeck’s Arch Surg 2012; 397: 917-925.
- Michalski CW, et al: Br J Surg 2007; 94: 265-273.
- Gleisner AL, et al: Cancer 2007; 110: 2484-2492.
- Chen KT, et al: Ann Surg Oncol 2014; 21: 662-669.
- Rose JB, et al: Ann Surg Oncol 2014; 21: 1530-1537.
- Hammel P, et al: Final results of the international phase III LAP 07 study. ASCT Meeting Abstracts 2013; 31: LBA4003.
InFo ONCOLOGY & HEMATOLOGY 2014; 2(7): 10-13.