Neuroendocrine tumors (NET) are not that rare. The prevalence of these tumors in the gastrointestinal tract is exceeded only by colon carcinoma. Often NETs are not detected until the late stages due to subtle symptoms and are often already in a palliative, inoperable stage. It is therefore important to think about these tumors. Many new options for surgical, local-interventional, and systemic therapy have been introduced in recent years. Due to the high degree of complexity in diagnosis and treatment of these tumors, collaboration in a specialized team of gastroenterologists, surgeons, pathologists, radiologists, nuclear medicine specialists, endocrinologists and oncologists is desirable.
Neuroendocrine tumors (NET) have a relatively low but increasing incidence of 4-5 new cases / 100 000 population per year [1]. However, the prevalence, i.e., the number of patients suffering from gastrointestinal neuroendocrine tumors (GEP-NET), is much higher and is surpassed in the gastrointestinal tract only by colon carcinoma. In addition, the incidence of all GEP-NET except appendiceal carcinoids is increasing [2]. In practice, GEP-NET are more common than expected. This short overview summarizes the most important facts in the diagnosis, the different forms of propagation diagnostics, the most important therapeutic measures as well as in the aftercare. The summary is based on the current guidelines of the European Neuroendocrine Tumor Society(www.enets.org).
Classification of GEP-NET
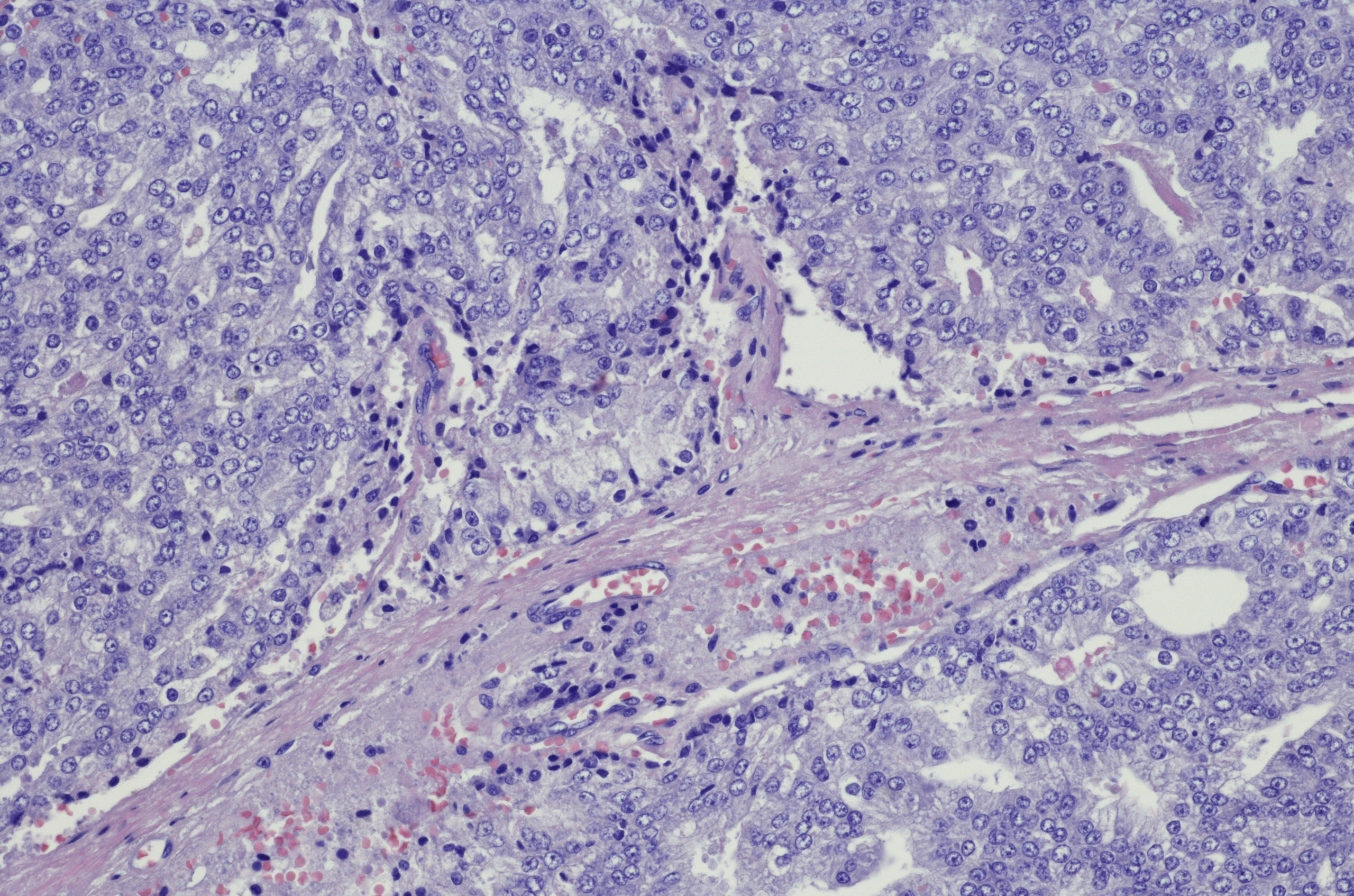
Neuroendocrine tumors are principally classified according to the location of the primary tumor and the number of mitoses (Ki67). The mitotic rate is one of the most important prognostic factors and divides these tumors into G1 (Ki67 ≤ 2%), G2 (Ki67 3-20%), and G3 (Ki67 >20%), with the G3 stage corresponding to a less differentiated, often aggressive neuroendocrine carcinoma compared to the better differentiated neuroendocrine tumors [3,4].
Gastrointestinal neuroendocrine tumors can occur at any site in the digestive tract. Depending on the location of the primary tumor, the characteristics and form of the metastasis are different and require a correspondingly different workup. The incidence of gastric NET (g-NET) has been steadily increasing in recent years and accounts for approximately 8% of GEP-NET [5]. Reasons for this are certainly the widespread introduction of gastroscopy and possibly the frequent use of proton pump inhibitors [6].
The g-NET are divided into three subgroups, the most common type I may be associated with atrophic gastritis, is usually well differentiated, rarely metastasizes, and has a good prognosis. Subtypes II and III are less common, metastasize more frequently, and (especially subtype III) have a poorer prognosis [5]. The workup and prognosis of these gastric tumors must be adjusted according to type and size. Duodenal NET (d-NET) are rare (1- 2% of GEP-NET), occur mostly in the first two-thirds of the duodenum, are usually relatively small, rather well differentiated, and nevertheless metastasize locally to lymph nodes in 40-60% [7]. The incidence of pancreatic GEP-NET (p-NET) ranges from 2 – 3/100 000 people per year [8]. Early signs of malignancy are often absent in p-NET. These patients present to the physician with local compression or signs of advanced metastasis (especially to the liver). For this reason, more than 50% of patients with p-NET already have distant metastasis.
An overall rare but typical underlying disease of p-NET (also of d-NET) is multiple endocrine neoplasia type I (MEN I).
Approximately 20% of patients with MEN1 suffer from p-NET with increasing incidence with age [8]. Likewise, patients with Von Hippel-Lindau disease, among other malignancies, are more likely to develop p-NET.
Jejunal and ileal NETs are the most common GEP NETs, accounting for up to 30% of all cases [9]. Small bowel NETs, similar to p-NETs, have a tendency to be already metastatic at diagnosis. Generally, local lymph nodes and later the liver are often affected. NET of the colon occur in approximately 7% of all GEP-NET and generally have the worst prognosis [10]. Similar to the previously described tumors, colon NETs are usually very advanced at diagnosis and metastasize locally, peritoneally, and to the liver. Rectal NET, on the other hand, are often found relatively early. This due to early symptomatology, such as pain or blood ab ano. For this reason, among others, metastases in rectal NET are rare [10]. Overall, GEP-NET biology is shown to vary depending on localization and by differentiation. Therefore, diagnosis, therapy and follow-up must be adapted to the primary localization.
Diagnostic workup and imaging
Depending on the localization, the clarification of GEP-NET differs. Diagnosis is made by histological confirmation [11]. Fine needle aspiration for cytological workup is often insufficient. Mandatory markers for diagnosis are synaptophysin and chromogranin A. The degree of differentiation (G1-G3) must also be determined. Additional markers, e.g., individual somatostatin receptors, are not mandatory but are of interest, e.g., for further therapeutic steps.
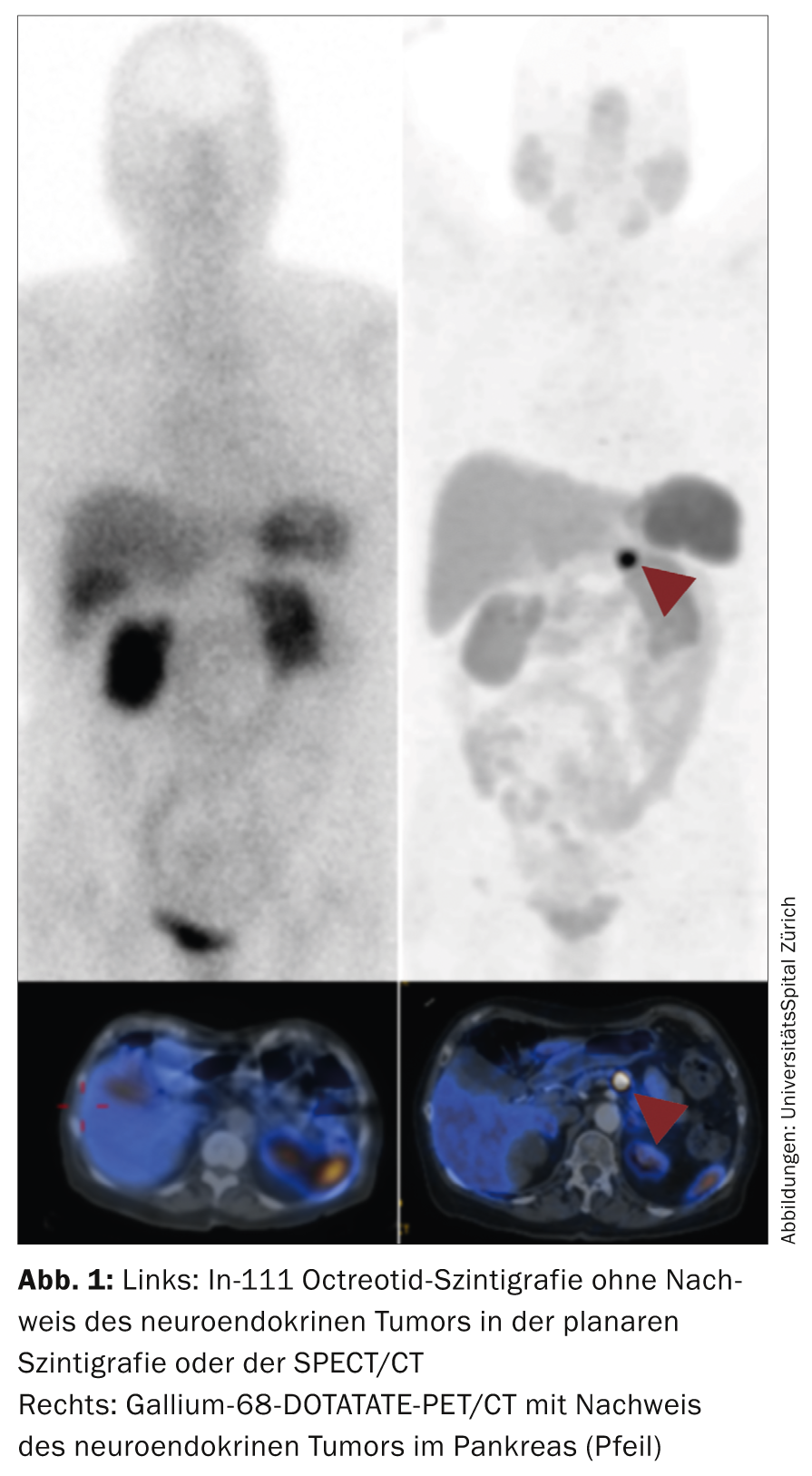
Once the diagnosis has been made, staging is performed [12]. Staging, along with grading, is the most important prognostic marker for the patient. Methods used include ultrasound (including endoscopic ultrasound), often contrast-enhanced CT, MRI, and functional methods such as octreotide scintigraphy and gallium-68 DOTATATE/DOTATOC PET/CT and fluorine-18 deoxyglucose (FDG) PET/CT. The domain of ultrasound is mainly the visualization of liver metastases with a sensitivity and specificity around 90%. Endoscopic ultrasound (EUS) is particularly used for pancreatic neuroendocrine tumors (e.g., pancreatic insulinomas or nonfunctional pancreatic tumors). The specificity/sensitivity is over 90%. Duodenal tumors may be more poorly visualized by EUS. The detection rate of these tumors by EUS is only around 60%. Contrast-enhanced CT is the most widely used method for staging GEP-NET. It is important to perform an arterial phase especially in liver metastases. This increases the detection rate of liver metastases to approximately 80%. However, sensitivity is somewhat lower (around 70%), especially for p-NET and soft tissue metastases. These tumors are the typical domain of MRI examination. MRI has a sensitivity of more than 90% for p-NET in the pancreas. Further, liver MRI with liver-specific contrast agent is the investigation of choice to find liver metastases. With a detection rate of up to 95%, liver MRI is the most sensitive method for liver metastases. Functional methods are typically used for propagation diagnostics and to measure somatostatin receptor expression (especially SSTR2) [13].
Octreotide scintigraphy, which was used until recently and has relatively high radiation exposure and acquisition protocols over several days, is now being replaced by gallium-68 DOTATATE/DOTATOC PET/CT. PET examination shows significantly lower radiation (about 30%) and much improved resolution compared to the scintigraphic method. In many NET centers, scintigraphy has been completely replaced by gallium-68 DOTATATE/DOTATOC PET/CT (Fig. 1) .
FDG-PET/CT is used for dedifferentiated carcinomas (G3), although even higher-grade G2 tumors may visualize better on FDG-PET. Patients with functional tumors with carcinoid symptoms must have echocardiography at least once to rule out cardiac involvement [14].
Biochemical markers
GEP-NET, especially “midgut” tumors, can produce and secrete functionally active peptides. However, the rate of clinically functional vs. nonfunctional tumors is only about 1:10. So-called “Foregut” and “Hindgut” tumors are somewhat less frequently functional. Measurement of these peptides in urine or blood can be used as a progression maker if appropriate symptoms are present [15].
The standard test is the 24h measurement of 5-indoleacetic acid (5-HIAA) in urine. In the presence of functional NET symptoms, the rates for sensitivity and specificity resp. at 70 and 90%. The 5-HIAA may also be elevated by inflammatory bowel disease and thus have a false-positive effect on the measurement result. Likewise, medications, e.g. chemotherapeutic agents or centrally acting substances such as tricyclic antidepressants, can influence the readings of the 24h urine collection and must be asked about.
Other tests, such as the fasting test for insulinoma or the secretin test for gastrinoma, belong in the hands of an experienced specialist (for example, a specialized endocrinology unit). Chromogranin A (CgA) is another important plasmatic biochemical marker of NET. CgA is formed in the neurosecretory granules of tumor cells and can be secreted equally in functional and nonfunctional NET. It should be noted that CgA may be falsely elevated specifically with concomitant use of proton pump inhibitors. Other factors that may increase CgA are: Renal insufficiency, Parkinson’s disease, untreated hypertension, pregnancy, glucocorticoids, chronic A-type gastritis, and inflammatory bowel disease. Nevertheless, CgA has been shown to be more accurate than urinary 5-HIAA measurement or the commonly performed Neuron Specific Enolase (NSE) [15].
Therapy of GEP-NET
Therapy of neuroendocrine tumors is complex and should be discussed and decided in a multidisciplinary manner. A tumor board with the presence of different diagnostic, surgical and medical disciplines should decide at least once on the diagnosis, therapy and follow-up of a patient with NET.
The first priority of any therapy is to aim for surgery (as long as it seems reasonable). The goal of surgery should be, whenever possible, the complete removal of the tumor. Furthermore, locally symptomatic tumors should be treated surgically, e.g., in case of impending illeus or blood ab ano. Cytoreductive therapy may be discussed in certain cases in a palliative setting with high tumor burden. However, this must be discussed in detail with the patient in view of the palliative background, the sometimes high morbidity of the intervention, and the often slowly progressing disease.
Patients with pronounced functional symptoms should be screened perioperatively with Sandostatin, possibly H1/H2 blockers, and cortisone [16]. It should also be noted that catecholamine can lead to an increased release of hormones.
In patients with high tumor burden, interventional procedures (e.g., embolization) may result in rapid changes in blood pressure. This is particularly important in patients with cardiac involvement of carcinoid syndrome. If no surgical intervention is possible, local-interventional or systemic therapies are used in principle.
Local-interventional particle embolization, chemoembolization, and radioembolization are used. However, classical bland embolizations are now performed in NET only in small numbers. This is mainly because of the high rate of side effects (due to the direct effect of severe local hypoxia and necrosis). Likewise, chemoembolization for NET often leads to severe symptoms (mainly post-embolization syndrome), especially in advanced, large liver tumors. Radioembolization is an elegant therapy with generally few side effects in the hands of experienced specialists. Local infusion of radioactive microspheres typically loaded with yttrium-90, a pure β-emitter, deposits local radiation up to more than 500 grays in the liver metastases in a single session. This due to the radiation properties of yttrium-90 and the typically high selective arterial perfusion of NET metastases in the liver (Fig. 2).
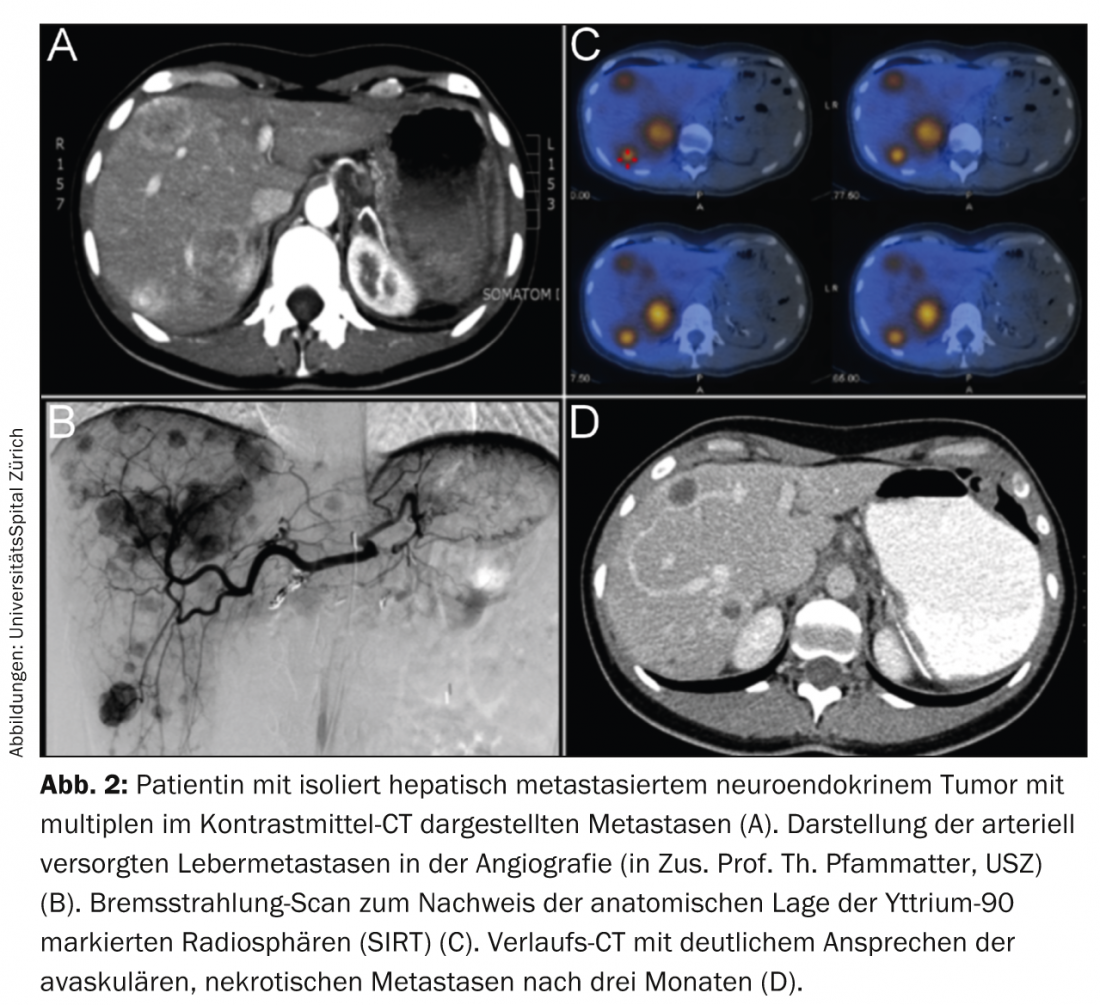
Because of the low rate of side effects of correctly performed outpatient radioembolization, chemoembolization and especially embolization have been almost completely replaced [17]. However, this therapy is only performed regularly at a few centers in Switzerland, e.g. the ENETS Center of Excellence at the University Hospital Zurich.
If surgical or local systemic therapy is not an option, palliative systemic therapy should be discussed at the tumor board. For nonpancreatic, well-differentiated “midgut” tumors, therapy with somatostatin analogues is the standard as the first line of therapy. In principle, two different long-acting therapeutic agents are available. Octreotide-LAR demonstrated prolonged progression-free survival in functionally active and inactive NET in a placebo-controlled trial (PROMID trial) in well-differentiated “midgut” tumors [18]. Analysis of the long-term data from the PROMID trial also showed a positive trend by octreotide on prolonged overall survival despite successful cross-over to the treatment arm (octreotide) after progression [19].
Data from lanreotide were also recently presented, which also showed a significant improvement in progression-free survival [20]. These preparations were not directly compared with each other, and which of these preparations can be used in which indication remains to be seen in clinical practice. At most, lanreotide will be used in more advanced tumors with higher tumor burden due to the design of the Clarinet trial. In pancreatic, well-differentiated (few “intermediate grade”) tumors, everolimus demonstrated prolonged progression-free survival and is approved as first-line Afinitor® in p-NET [21].
Everolimus in combination with octreotide may also be considered in additional NET e.g. lung and colon due to the positive impact on progression-free survival in the RADIANT-2 trial, narrowly missing statistical significance [22]. Likewise, Sutent® shows a prolongation of progression-free survival as well as overall survival in patients with well-differentiated NET of the pancreas [23]. Both drugs are options in patients with well-differentiated p-NET. However, the side effects of these therapies are sometimes significant and should be performed by an experienced specialist. The aforementioned systemic therapies are mainly used in well-differentiated GEP-NET.
For higher proliferative G2 tumors, combined chemotherapies are classically used. In particular, 5-fluorouracil-based therapies in combination with streptozotocin or Temodal® are used [24,25]. These chemotherapies are generally relatively toxic, but can be used with good success by experienced oncologists, especially in patients with high tumor load, symptoms, and relatively rapid growth. Patients with highly proliferative, dedifferentiated large- or small-cell neuroendocrine carcinomas often benefit from combination chemotherapy with cisplatin and etoposide [26]. Patients with high expression of somatostatin receptors on octreotide scintigraphy or gallium-68 DOTATATE/DOTATOC PET/CT can be evaluated for peptide-based radionuclide therapy. This systemically administered radionuclide therapy irradiates SSTR2-positive tumors by binding and uptake of radiolabeled somatostatin analogs. This therapy is currently being studied in a phase III trial vs. octreotide-LAR. In phase II trials, this therapy was shown to be very effective with a very low rate of side effects [27]. In particular, the frequently mentioned renal insufficiency occurs in less than 1% of patients. This therapy is offered by some centers in Switzerland, e.g. the ENETS Center of Excellence at the University Hospital Zurich or the University Hospitals Basel and Bern. Here, the University Hospital Basel has taken on a pioneering role. New therapeutic approaches are investigating new combinations of the aforementioned therapy and exploring new avenues such as reducing carcinoid symptomatology [28]. Second-generation somatostatin analogues (pasireotide Signifor®) did not show improved symptom control in a phase III trial, but did show a five-month increase in progression-free survival compared with octreotide-LAR [29].
In general, there is a wide variety of different therapies in GEP-NET and these must be adapted to the location of the primary tumor and its differentiation. This is one of the reasons why patients with NET should be discussed at a specialized tumor board.
Follow-up of patients with NET
Follow-up of patients with NET varies depending on the primary tumor and tumor differentiation. In general, benign insulinomas after removal, rectal carcinoids after removal, and early appendiceal carcinoids do not require follow-up. Type I gastric NET need annual endoscopy. In general, G1 tumors need monitoring by imaging and laboratory every twelve months, G2 tumors every six months, and G3 carcinomas every three months. Recommended is the successful method of staging [30].
Prof. Dr. med. Niklaus G. Schaefer
Literature:
- Yao JC, et al: One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008; 26(18): 3063-3072.
- Tsikitis VL, Wertheim BC, Guerrero MA: Trends of Incidence and Survival of Gastrointestinal Neuroendocrine Tumors in the United States: A Seer Analysis. J Cancer 2012; 3: 292-302.
- Rindi G, et al: TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Archive 2006; 4; 395-401.
- Rindi G, et al: TNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Archive 2007; 4; 757-762.
- Ruszniewski P, et al: Well-differentiated gastric tumors/carcinomas. Neuroendocrinology 2006; 84: 158-164.
- Lawrence B, et al: A clinical perspective on gastric neuroendocrine neoplasia. Curr Gastroenterol Rep 2011 Feb; 13(1): 101-109.
- Robert T, et al: Well-differentiated duodenal tumor/carcinoma (Excluding gastrinomas). Neuroendocrinology 2006; 84: 165-172.
- Falconi M, et al: Well-Differentiated Pancreatic Nonfunctioning Tumors/Carcinoma. Neuroendocrinology 2006; 84: 196-211.
- Eriksson B, et al: Consensus Guidelines for the Management of Patients with Digestive Neuroendocrine Tumors – Well-Differentiated Jejunal-Ileal Tumor/Carcinoma. Neuroendocrinology 2008; 87: 8-19.
- Ramage JK, et al: Consensus Guidelines for the Management of Patients with Digestive Neuroendocrine Tumors: Well-Differentiated Colon and Rectum Tumor/Carcinoma. Neuroendocrinology 2008; 87: 31-39.
- Klöppel G, et al: ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Towards a Standardized Approach to the Diagnosis of Gastroenteropancreatic Neuroendocrine Tumors and Their Prognostic Stratification. Neuroendocrinology 2009; 90: 162-166.
- Sundin A, et al: ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Radiological Examinations. Neuroendocrinology 2009; 90: 167-183.
- Dik J, et al: ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Somatostatin Receptor Imaging with 111 In-Pentetreotide. Neuroendocrinology 2009; 90: 184-189.
- Plöckinger U, et al: ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Echocardiography. Neuroendocrinology 2009; 90: 190-193.
- O’Toole D, et al: ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Biochemical Markers. Neuroendocrinology 2009; 90: 194-202.
- Akerström G, et al: ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Pre- and Perioperative Therapy in Patients with Neuroendocrine Tumors. Neuroendocrinology 2009; 90: 203-208.
- Kennedy A, et al: Radioembolization for Unresectable Neuroendocrine Hepatic Metastases Using Resin 90Y Microspheres: Early Results in 148 Patients. Am J Clin Oncol 2008; 31: 271-279.
- Rinke A, et al: Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 2009 Oct 1; 27(28): 4656-4663.
- Arnold R, et al: Placebo controlled, double blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors (PROMID): Results on long-term survival. J Clin Oncol 2013; 31 (suppl; abstr 4030).
- www.esmo.org/Conferences/Past-Conferences/European-Cancer-Congress-2013/News/Phase-III-Trial-Results-Favour-Lanreotide-Therapy-in-Patients-with-Gastroenteropancreatic-Neuro-Endocrine-Tumours.
- Yao JC, et al: Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011 Feb 10; 364(6): 514-523.
- Pavel ME, et al: Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet 2011 Dec 10; 378(9808): 2005-2012.
- Raymond E, et al: Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011 Feb 10; 364(6): 501-513.
- Engstrom PF, et al: Streptozocin plus fluorouracil versus doxorubicin therapy for metastatic carcinoid tumor. J Clin Oncol 1984 Nov; 2(11): 1255-1259.
- Strosberg JR, et al: First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer 2011 Jan 15; 117(2): 268-275.
- Fazio N, Spada F, Giovannini M: Chemotherapy in gastroenteropancreatic (GEP) neuroendocrine carcinomas (NEC): a critical view. Cancer Treat Rev 2013 May; 39(3): 270-274.
- Kwekkeboom DJ, et al: Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol 2008 May 1; 26(13): 2124-2130.
- http://telotristat-telestar.com/what-is-the-telestar-trial.php.
- Wolin EM, et al: A multicenter, randomized, blinded, phase III study of pasireotide LAR versus octreotide LAR in patients with metastatic neuroendocrine tumors (NET) with disease-related symptoms inadequately controlled by somatostatin analogs. J Clin Oncol 2013; 31 (suppl; abstr 4031).
- Arnold R, et al: ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Follow-Up and Documentation. Neuroendocrinology 2009; 90: 227-233.
InFo Oncology & Hematology 2014; 2(5): 14-18.