There are increasing signs of success in the treatment not only of rheumatoid arthritis (RA), but also of psoriatic arthritis (PsA). At the ACR Congress in San Diego, researchers were able to show that targeted treatment (“tight control”), similar to that used in RA, produces better results in terms of joint symptoms than the standard variant. In addition, there are new findings on the IL17 receptor antibodies brodalumab and secukinumab.
(ag) Laura C. med. Coates of the University of Leeds presented the results of her randomized controlled trial, TICOPA [1], which examined what outcomes can be achieved with tight control (TC, or targeted treatment) in early psoriatic arthritis (PsA).
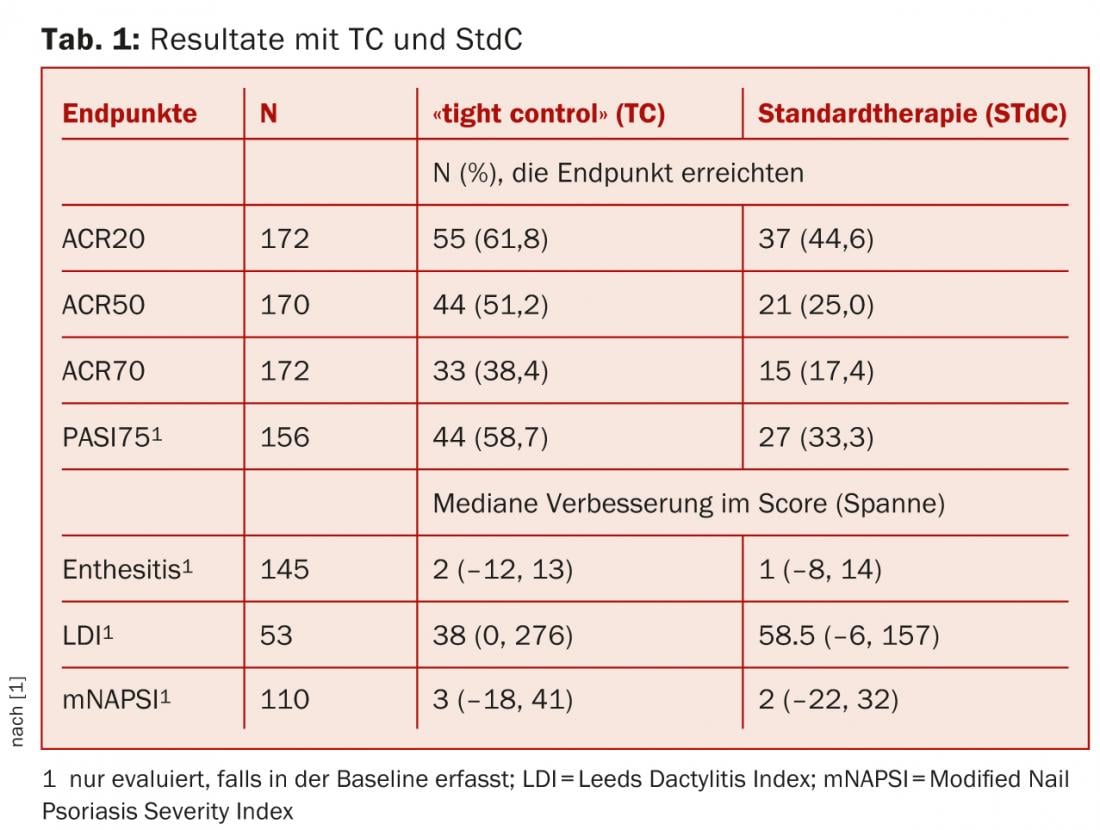
206 previously untreated patients with early PsA (symptom duration <24 months) were studied. They were randomly divided into two arms: one received TC (assessment every four weeks), the other standard therapy (StdC, assessment every 12 weeks) for 48 weeks.
- The 101 patients in the TC group followed a strict treatment protocol with escalation of therapy if they did not meet minimal disease activity (MDA) criteria: All participants started on methotrexate with a rapid escalation to 25 mg after six weeks with good tolerance. After twelve weeks, they were switched to combination DMARDs if they had not achieved MDA. After a further 12 weeks, they stepped up therapy to either anti-TNFs for ≥3 sensitive or swollen joints (defined by UK NICE guidelines) or to an alternative DMARD in combination with methotrexate for those who were not in MDA but had <3 active joints (defined by UK NICE guidelines).
- The 105 patients in the StdC group were treated by a rheumatologist, with no protocol or restrictions.
- The primary endpoint was ACR20 response at week 48, and secondary endpoints included ACR50 and ACR70, and PASI75 at week 48.
Better results with protocol group
At week twelve, twelve patients dropped out (5 TC, 7 StdC) and twelve were no longer available for follow-up (6 TC, 6 StdC). Participants had a median age of 45 years in both arms, 52% were men, 48% women, and 71% showed polyarthritis.
With TC, those treated were significantly more likely to achieve an ACR20 by week 48 than the comparison group (p=0.0392). Likewise, ACR50 (p=0.0081) and ACR70 (p=0.0058) were achieved more frequently (Table 1).
The most common side effects were nausea, liver function test abnormalities, and infections (i.e., normal cold). Adverse events were observed in 88% of patients (97% TC vs. 80% StdC). There were 33 severe cases (25 TC, 8 StdC) in 20 patients (14 TC, 6 StdC). No deaths or unexpected serious adverse events occurred.
“Targeted, protocol-directed treatment leads to significantly better joint and skin outcomes in newly diagnosed PsA patients without leading to unexpected severe side effects,” Dr. Coates concluded.
Promising news on IL17 receptor antibodies
Furthermore, two new IL17 receptor A antibodies are currently being investigated: brodalumab [2] and secukinumab [3].
Brodalumab was tested in 113 PsA patients and at two doses (140 or 280 mg every two weeks). Placebo was given to 55 patients. 37 and 39% of patients on the monoclonal antibody achieved an ACR20 at week twelve, respectively, compared to 18% in the placebo group (p<0.05). Improvements continued to increase through week 24. The most common side effects included mild infections, arthralgias, and oropharyngeal pain. Severe neutropenia (≥Grade 2) did not occur.
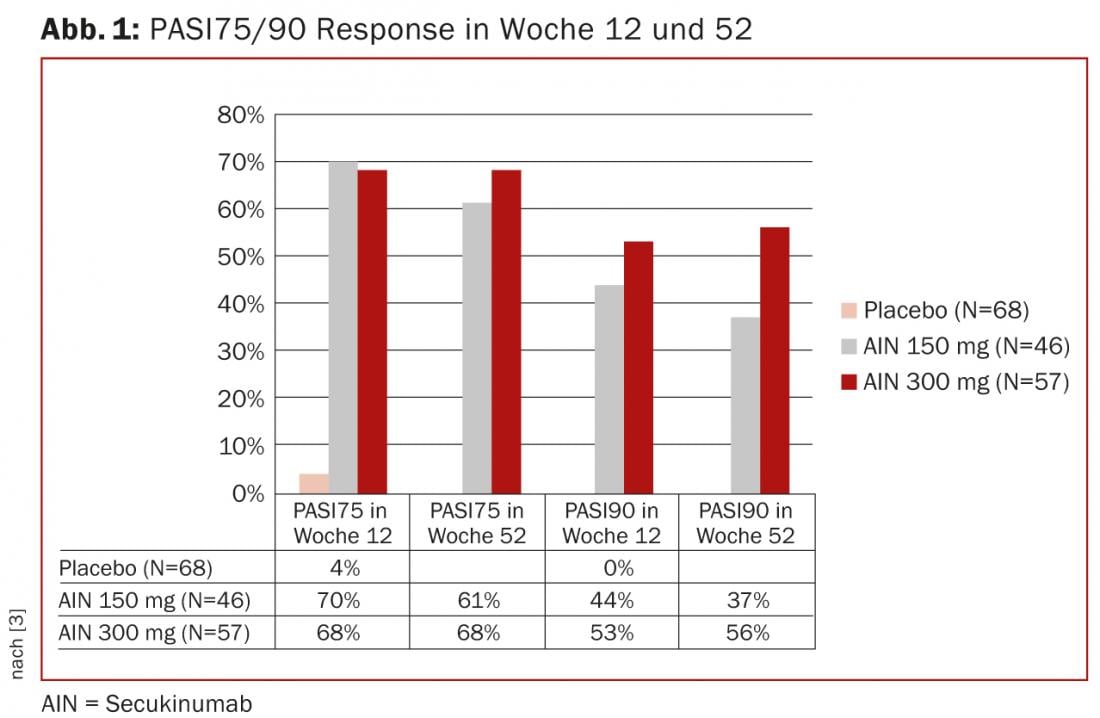
The subanalysis of a phase III study in plaque psoriasis showed a significantly better PASI75 (150 mg 70%, 300 mg 68%, placebo 4%) and PASI90 (150 mg 44%, 300 mg 53%, placebo 0%) in 171 patients treated with secukinumab after 12 weeks on the one hand, and a better PASI75 (150 mg 61%, 300 mg 68%) and PASI90 (150 mg 37%, 300 mg 56%) after 52 weeks on the other hand. (Fig. 1). On the other hand, functionality (HAQ-DI) was also increased compared to placebo.
Source: ACR/ARHP Annual Meeting, October 26-30, 2013, San Diego.
Literature:
- Coates LC, et al: Results Of a Randomised Controlled Trial Comparing Tight Control Of Early Psoriatic Arthritis (TICOPA) With Standard Care: Tight Control Improves Outcome. ACR Abstract #814.
- Genovese M, et al: Clinical Response To Brodalumab, An Anti-Interleukin-17 Receptor Antibody, In Subjects With Psoriatic Arthritis. ACR Abstract #817.
- Gottlieb A, et al: Secukinumab Shows Substantial Improvement In Both Psoriasis Symptoms And Physical Functioning In Moderate-To-Severe Plaque Psoriasis Patients With Psoriatic Arthritis: A Subanalysis Of A Phase 3, Multicenter, Double-Blind, Placebo-Controlled Study. ACR Abstract #319.
CONGRESS SPECIAL 2014; 6(1): 7-8











