The management of hidradenitis suppurativa remains a clinical challenge. A combination of different therapeutic modalities is often required to achieve a sufficient degree of disease control. One study demonstrated that the combined use of adalimumab in parallel with surgical excision is effective and safe. To date, adalimumab is the only approved biologic in HS, but several other systemic anti-inflammatory drug candidates are currently being investigated in clinical trial programs.
Hidradenitis suppurativa (HS) is a chronic relapsing, progressive, immune-mediated disease. The nodules, abscesses, and fistulas occur preferentially axillary, inguinal, and anogenital and can be very painful. If not treated early enough, inflammation can develop into irreversible tissue damage, explained Prof. Dr. med. Falk Bechara, Senior Physician Dermatology at the University Hospital of the Ruhr University Bochum (D) [1]. The following criteria can be used to diagnose HS [2–4]:
- characteristic morphology of the lesions,
- characteristic distribution of lesions,
- Chronicity and recurrent manifestations
If a patient meets all three of these diagnostic criteria, it is HS with a sensitivity of 90% and a specificity of 97% [2–4]. HS severity is most often assessed according to the Hurley classification [2–4] (mild=stage I, moderate=stage II, severe=stage III). Regarding pathophysiology, there are still many open questions, the speaker said. It appears that a great many inflammatory mediators and other parameters are upregulated in HS patients.
Nowadays, the main treatment options for HS are considered to be: systemic antibiotics, surgical intervention, biologics. Prof. Bechara added that usually several of these therapeutic approaches are combined [1].
OP: Paradigm shift regarding approach
Surgical intervention is usually required for patients in Hurley stages II or III [11]. The primary goal is to remove fistula tracts and prevent recurrent occurrence of individual lesions [12,13]. Complete anatomic phrophylactic resection for initial fistulas as practiced in the past would not be done nowadays, Prof. Bechara explained [1]. Instead, he said, the current focus is on identifying irreversible tissue damage. The point, he said, is to target resection of irreversible fistulizing scarring stages rather than simply resecting all inflammatory disease manifestations. Flat lesions can also be operated on flat and combined with medication, and a radical procedure is not always necessary, the speaker elaborated.
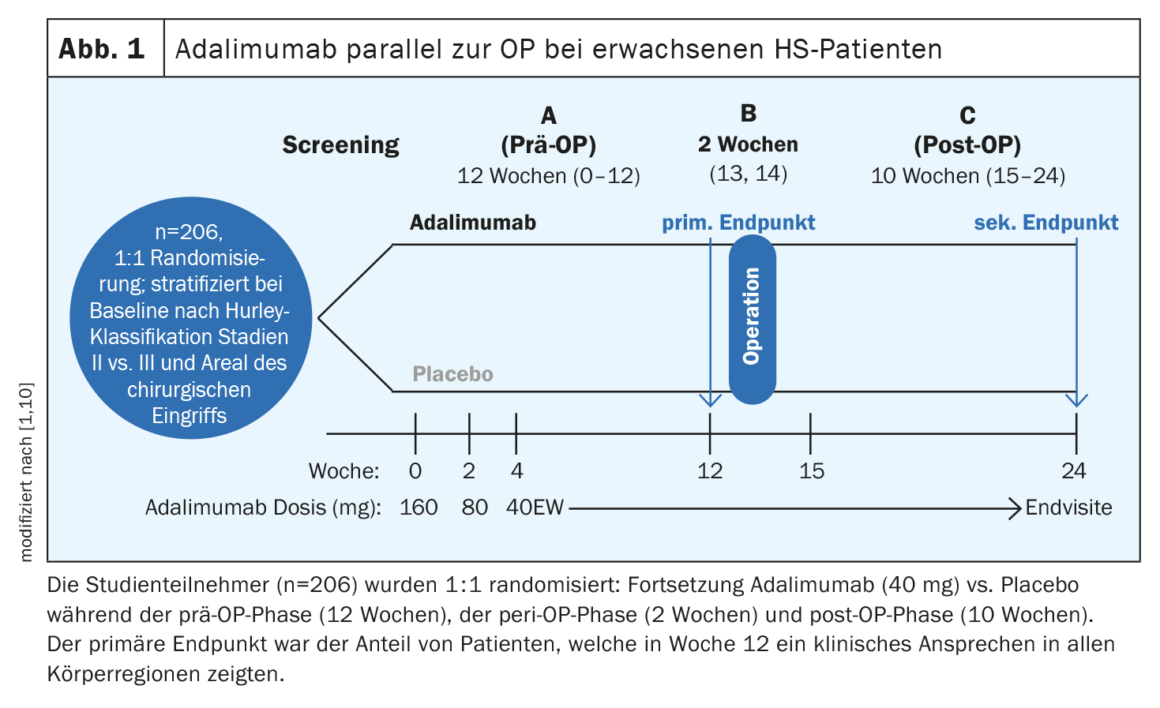
Adalimumab in parallel with surgery proved to be effective and safe
Apart from adalimumab, no other biologic has yet cleared the approval hurdles. “We have a preparation that works and we are working with it, but there is plenty of room for improvement,” the speaker summarized [1]. In the PIONEER I and II studies, HiSCR50 response rates of 42% and 59%, respectively, were achieved with adalimumab, whereas the corresponding values with placebo were 26% and 28%, respectively [5]. Before a surgical procedure, the question often arises as to whether or not to discontinue the biologic. In a study published in 2021, adalimumab was shown to be effective in combination with major surgery followed by secondary healing, without the need to interrupt treatment before surgery [10]. The SHARPS (Safety and Efficacy of Adalimumab for Hidradenitis Suppurativa Peri-Surgically) study was a randomized, double-blind, placebo-controlled phase IV trial of adalimumab in combination with surgery (Fig. 1) . A total of 103 patients were randomized to adalimumab and placebo, respectively. The mean age (SD) was 37.6 (11.3) years, and 51% of participants were women. A clinical response to HS in all body regions was achieved at week 12 by 48% of study participants on adalimumab and 34% on placebo (p=0.049). Treatment-related adverse events were reported in 72% in the adalimumab arm and 69% in the placebo arm. No increased risk of postoperative wound infection, complications, or bleeding was observed with adalimumab compared with placebo.
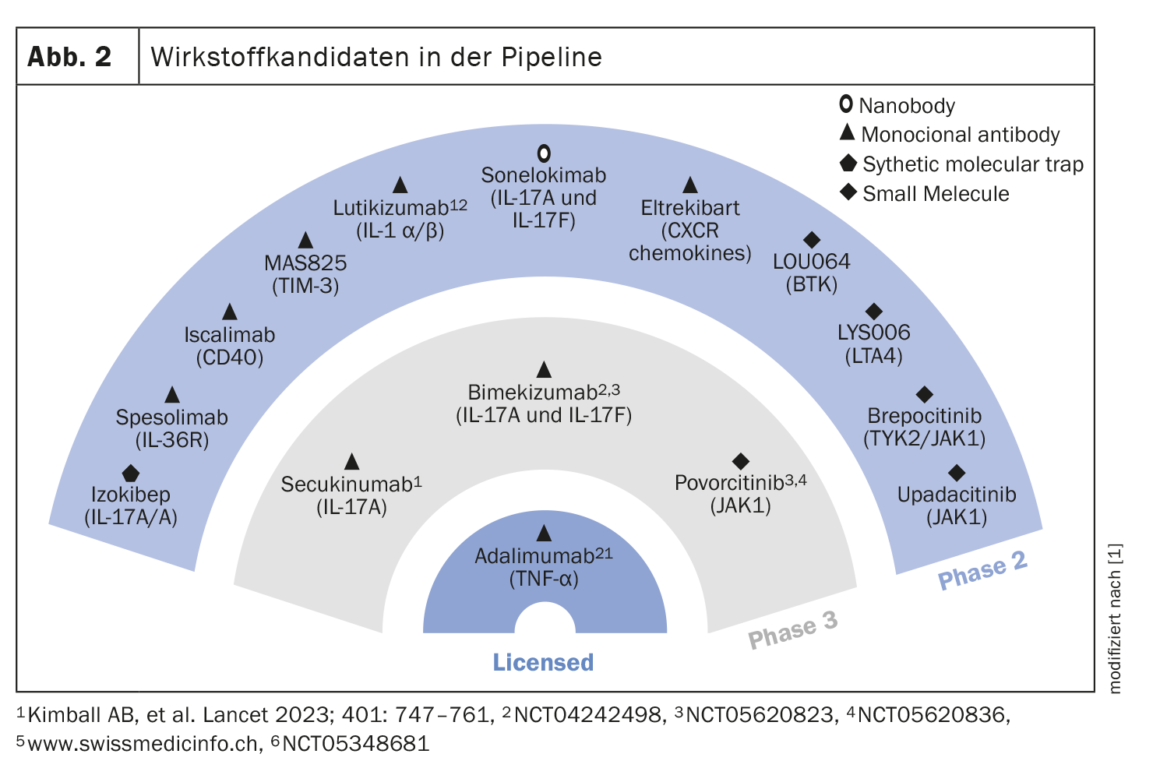
Further drug candidates in the pipeline
The drug candidates currently under investigation in Phase II and III are shown in Figure 2. Two clinical trials demonstrated that the IL17A inhibitor secukinumab at two doses (300 mg every 2 weeks and every 4 weeks, respectively) showed stable response rates up to week 52 with 54.8% and 55.3% in SUNSHINE and 63.4% and 58.6% in SUNRISE, respectively [6]. The dual IL17A/F inhibitor bimekizumab (320 mg q2w) met the primary endpoint of HiSCR50 in the BE HEARD I trial compared with placebo, and it also reached the significance level in BE HEARD II. A monoclonal antibody targeting IL36R, is spesolimab [7]. In a proof-of-concept study, spesolimab was shown to be very effective at week 12, particularly with regard to draining fistulas. The loading dose was 1200 mg (i.v.) at weeks 0,1 and 2. In maintenance therapy, the same dose was administered at weeks 4,6,8 and 10. Another drug candidate being explored for use in HS is Izokibep. Its target is the IL17A homodimer. The small molecule size allows for better tissue penetration. In a non-placebo-controlled open-label study of 30 patients, a proportion of 71% achieved a HiSCR50 response at week 12 with Izokibep 160 mg [8]. A selective JAK1 inhibitor for which phase II data are available for the HS indication area is povorcitinib. At the highest dose (90 mg), more than 80% of 9 patients achieved HiSCR50 at week 8 [9].
Congress: DDG Annual Conference
Literature:
- «Acne inversa: Medikamente, Messer, Mischung», Prof. Dr. med. Falk Bechara, DDG-Jahrestagung, 26.–29.04.2023.
- Johnston LA, et al.: Practical Guidelines for Managing Patients With Hidradenitis Suppurativa: An Update. J Cutan Med Surg 2022; 26(2_suppl): 2S–24S.
- Daxhelet M, et al.: Proposed definitions of typical lesions in hidradenitis suppurativa. Dermatology 2020; 236(5): 431–438.
- Revuz JE, Jemec GBE: Diagnosing hidradenitis suppurativa. Dermatol Clin 2016; 34(1): 1–5.
- Kimball AB, et al.: Two Phase 3 Trials of Adalimumab for Hidradenitis Suppurativa. NEJM 2016; 375: 422–434.
- Kimball AB, et al.: Secukinumab in moderate-to-severe hidradenitis suppurativa (SUNSHINE and SUNRISE): week 16 and week 52 results of two identical, multicentre, randomised, placebo-controlled, double-blind phase 3 trials. Lancet 2023; 401(10378): 747–761.
- Alavi et al. Spesolimab for Hidradenitis Suppurativa: a proof-of-concept study in patients with Hidradenitis suppurativa. AAD 2023; Poster 43019
- Papp K, et al.: Izokibep, a novel IL17A-inhibitor demnstrates HiSCR100 Responses in moderate-to-severe Hidradenitis suppurativa: week 12 results of open-label par A of a Phase 2b/3-study. AAD 2023; Late-breaking oral.
- Alavi A, et al.: Janus kinase 1 inhibitor INCB054707 for patients with moderate-to-severe hidradenitis suppurativa: results from two phase II studies. Br J Dermatol 2022; 186(5): 803–813.
- Bechara FG, et al.: Efficacy and Safety of Adalimumab in Conjunction With Surgery in Moderate to Severe Hidradenitis Suppurativa: The SHARPS Randomized Clinical Trial. JAMA Surg 2021; 156(11): 1001–1009.
- Schuch A, Absmaier-Kijak M, Volz T: Acne inversa/Hidradenitis suppurativa – Von der Pathogenese zur Therapie. Akt Dermatol 2019; 45: 277–287.
- Melendez Gonzalez MDM, Sayed CJ. Surgery is an essential aspect of managing patients with hidradenitis suppurativa. J Am Acad Dermatol 2020; 83(3): 979–980.
- Janse I, et al.: Surgical procedures in hidradenitis suppurativa. Dermatol Clin 2016; 34(1): 97109.
DERMATOLOGIE PRAXIS 2023; 33(3): 28–29