In certain tumor types and in malignancies with limited peritoneal carcinomatosis, it is possible to pursue a curative approach even at this stage of the disease by combining cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS/HIPEC). However, the indication must be made very carefully and selectively. Laparoscopy is usually recommended for definitive assessment of resectability in malignant tumors. In appendiceal tumors, colon carcinoma, and primary peritoneal mesothelioma mésothéliome, 5-year survival can thus be increased by CRS/HIPEC.
Peritoneal carcinomatosis is an advanced tumor manifestation of many gastrointestinal tumors, for example, appendix, colon, stomach, or ovary, but also tumors of the hepatobiliary system and pancreas. Primary peritoneal tumors, such as primary peritoneal mesotheliomas originating from the mesothelium of the peritoneum, are extremely rare. Some tumor types, for example low-malignant appendiceal tumors, are progressive but usually slow and confined to the abdomen; carcinomatosis in pancreatic or gastric carcinoma, on the other hand, is rapid, usually involves the lymphatic compartment, and is often refractory to therapy. Therefore, the range of primary tumor types and additional histologic subtypes (e.g., intestinal or signet-ring cell differentiation) does not allow generalization of therapeutic options and corresponding indications.
Principle and indication of cytoreductive surgery
The approach to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS/HIPEC) is curative rather than palliative. A strict and careful indication is therefore mandatory. In malignant tumors, the therapy is suitable for early or limited carcinomatosis and is not very useful as a last option. Trusting interdisciplinary cooperation and jointly developed concepts are therefore essential for therapy planning. Knowledge of tumor biology is also important; for example, carcinomatosis in tumors of the liver, biliary tract, or pancreas is not an indication for surgical intervention. Reasonably, CRS/HIPEC is useful for the treatment of limited peritoneal carcinomatosis in tumors of the appendix, colon, and primary peritoneal mesothelioma; CRS/HIPEC is also conceivable in ovarian cancer and highly selective in gastric cancer.
HIPEC is used to treat free or microscopic tumor cells remaining on the peritoneum after radical surgery, thus completing the result of the surgical procedure. The term “chemotherapy” is not really appropriate, despite the substances used, since HIPEC probably only completes the macroscopic result of surgery at the microscopic level.
In keeping with the curative approach, definitive assessment of resectability usually requires laparoscopy, and sometimes exploratory laparotomy. Here, the extent of peritoneal carcinomatosis is determined by determining the “Peritoneal Cancer Index” (PCI) [1]. The abdomen is divided into 13 quadrants and each is assigned a value between 0 and 3. Thus, the PCI is between 0 and 39. The decision to resect is only made if this can be performed radically (complete cytoreduction [CC]-score 0, no macroscopically visible tumor). Regarding long-term prognosis, PCI should not exceed a maximum value for certain tumor types. Contraindication for cytoreduction and thus also HIPEC is often a pronounced tumor involvement of the small bowel, which may make radical resection impossible. In such cases, the procedure should be discontinued, as it is not justifiable that, given the expected (poor) prognosis, the quality of life should be diminished by postoperative impairment.
Technical aspects
If the decision is made to resect, the tumor-involved peritoneum is removed (peritonectomy). Organ resections are performed sparingly and exclusively for tumor involvement. The exception is oncological resections if the primary tumor (e.g., in the colon or appendix) has not been previously removed. Resection is considered complete when there is no visible tumor left (CC-score 0), only then HIPEC is performed. Direct intra-abdominal application of cytostatic drugs achieves high concentrations in the abdomen while keeping systemic exposure low. Simultaneous heating of the chemotherapeutic carrier solution (dialysis fluid) to 42°C improves the penetration and, to some extent, the cytotoxic effect of the cytostatic drugs [2]. Mainly combinations of mitomycin C and doxorubicin, cisplatin, oxaliplatin and sometimes docetaxel are used. The temperatures vary from 41° to 43°. The application takes place during 30-90 minutes, depending on the substances used.
Side effects
The morbidity of the procedure depends mainly on the extent of cytoreduction. Renal insufficiency from HIPEC occurs extremely rarely, especially with good control of intraoperative diuresis. The rate of cytostatic-induced hemotoxicity is also low. A French multicenter study [3] and a systematic search of the current literature [4] show a postoperative complication rate of approximately 30% and a mortality of 3-5% with appropriate experience. Significant risk factors in the multivariate analysis in the French study were patient age, PCI and associated resection extent, and center experience [3,5]. HIPEC causes somewhat prolonged intestinal paralysis in almost all patients, so they are initially fed parenterally.
Special indications and results according to tumor type
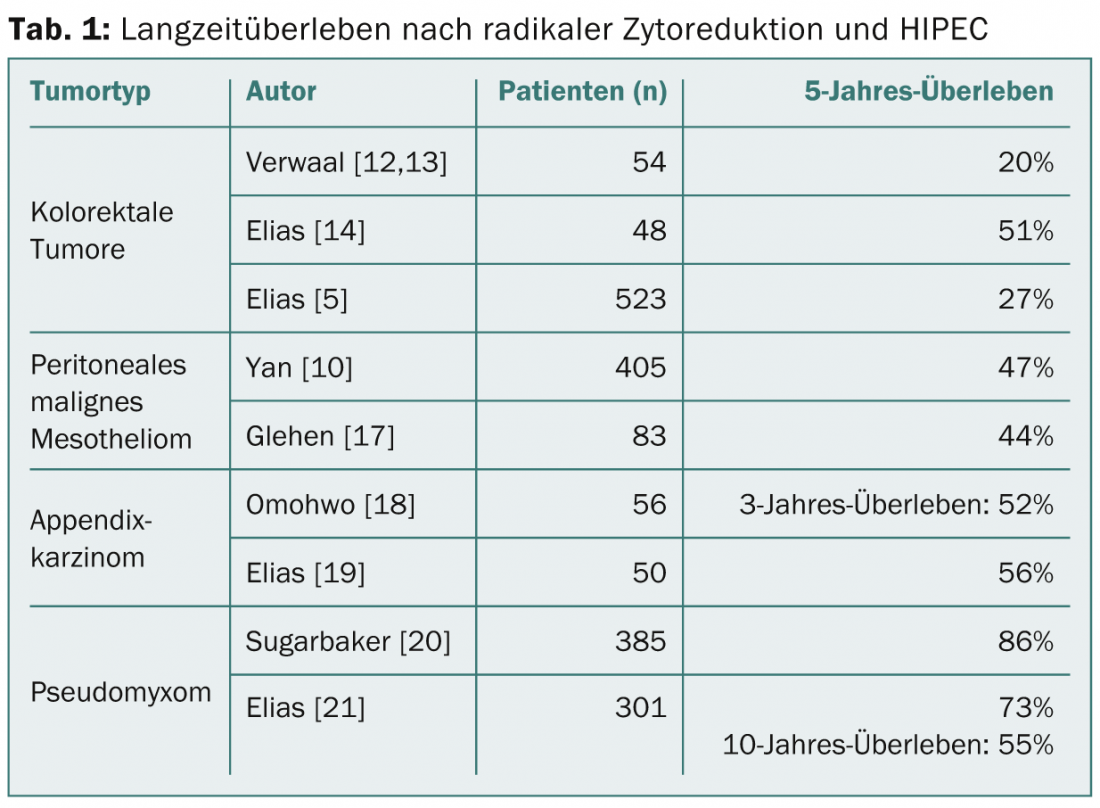
Pseudomyxoma and appendiceal tumors: Appendiceal tumors exhibit a high variation in histologic types. Peritoneal carcinomatosis can be very cell-poor and/or mucin-rich, but goblet cell carcinomas, adenocarcinomas, and signet ring carcinomas can also be found, increasing in aggressiveness in that order. The manifestation in the form of cell-poor, low-malignant mucinous masses (disseminated peritoneal adenomucinosis, DPAM) corresponds to the classic pseudomyxoma. In this disease, a 5-year survival of 85% can be achieved by CRS/HIPEC (Table 1) [6]. These excellent results are still evident over the course of 10-20 years [7]. For cell-rich tumors (peritoneal mucinous carcinomatosis, PMCA), 5-year survival is lower at 47%, but it is still higher than for colorectal tumors [8]. A worse prognosis is found in appendiceal tumors with signet ring cell differentiation.
It should be noted that the histology of the peritoneal carcinomatosis is relevant to prognosis, rather than the histology of the primary tumor. Based on current data, all appendiceal tumors with exclusively peritoneal involvement can be evaluated for CRS/HIPEC. However, especially in higher-grade tumors, systemic therapy is primarily required in case of extensive involvement; in the absence of response or extensive small bowel involvement, CRS/HIPEC is also not indicated in this case. This does not apply to classic pseudomyxoma, in which case CRS/HIPEC should be performed even if PCI is high.
Malignant peritoneal mesothelioma: Currently, there is no effective systemic treatment for primary peritoneal mesothelioma, and survival is limited [9]. A multicenter study of 401 patients with a median PCI of 20 showed a median survival of 53 months and a 5-year survival of 47% after CRS/HIPEC [10]. Negative predictive factors include PCI, lymph node involvement, and distant metastases [11]. Because of these promising survival data, CRS/HIPEC is a reasonable treatment option in this rare disease despite the lack of randomized trials.
Colorectal carcinoma: To date, only one prospective randomized trial exists that compared cytoreductive surgery and HIPEC with systemic chemotherapy. Verwaal et al. showed prolonged survival in patients with colorectal tumors in the long-term in 2003 as well as in 2008 [12,13]. This study is controversial today, on the one hand because of the insufficiently radical resection, on the other hand with regard to the now obsolete chemotherapy with 5FU only. In a non-randomized trial, CRS/HIPEC with oxaliplatin was compared with patients receiving modern systemic chemotherapy: in the patients after CRS/HIPEC, median survival improved from 24 to 63 months; in addition, 5-year survival increased from 13 to 51% [14]. Further analysis of multi-institutional experience over several years showed an overall 5-year survival of 27%, which was even higher with low PCI [5]. Single liver metastases are not an absolute contraindication; they can be resected simultaneously with good success, but only if peritoneal involvement is limited [15,16].
Several studies are currently underway to clarify the role of CRS/HIPEC in colorectal cancer compared with modern systemic therapy. Currently, CRS/HIPEC is reasonable in patients with limited involvement of the peritoneum (PCI <20), whereas the procedure should be discontinued in favor of palliative therapy in more advanced stages or when it cannot be performed radically (small bowel involvement). What remains to be discussed is the role of neoadjuvant/preoperative therapy leading to further patient selection. It is important to note that due to the insufficient resolution of imaging techniques, peritoneal involvement – and thus resectability – can often only be assessed during exploratory laparoscopy or laparotomy.
Conclusion and recommendation for practice
Treatment of peritoneal carcinomatosis by CRS/HIPEC is an effective therapeutic option for well-selected patients in good general health who usually have little chance of long-term survival without aggressive therapy. The prerequisite for CRS/HIPEC is radical resection (CCR-0), ideally early. The multimodality of treatment absolutely requires careful, interdisciplinary indication and cooperation.
Dr. med. Kuno Lehmann
Literature:
- Jacquet P, Sugarbaker PH: Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis: Kluwer Academic Publishers, Boston (MA); 1996.
- Ceelen WP, Flessner MF: Nat Rev Clin Oncol 2010; 7: 108-115.
- Glehen O, Elias D, Gilly F: Présentation du rapport de l’AFC. In : Carcinoses péritonéales d’origine digestive et primitives 2008.
- Chua TC, et al: Ann Surg 2009; 249: 900-907.
- Elias D, et al: J Clin Oncol 2010; 28: 63-68.
- Glehen O, et al: Cancer 2010; 116(24): 5608-5618.
- Sugarbaker PH: Lancet Oncol 2006; 7: 69-76.
- Elias D, et al: Eur J Surg Oncol 2010; 36: 456-462.
- Fizazi K, et al: J Clin Oncol 2003; 21: 349-354.
- Yan TD, et al: J Clin Oncol 2009; 27: 6237-6242.
- Yan TD, et al: Cancer 2011; 117: 1855-1863.
- Verwaal VJ, et al: Ann Surg Oncol 2008; 15: 2426-2432.
- Verwaal VJ, et al: J Clin Oncol 2003; 21: 3737-3743.
- Elias D, et al: J of Clin Oncology 2009; 27: 681-685.
- Chua TC, et: Eur J Surg Oncol 2009; 35: 1299-1305.
- Elias D, et al: Prognostic Similarities and Differences in Optimally Resected Liver Metastases and Peritoneal Metastases From Colorectal Cancers. Ann Surg 2014 Feb. [Epub ahead of print].
- Tong L, et al: Ophthalmology 2009; 116: 572-579.
- Omohwo C, et al: J Am Coll Surg 2009; 209: 308-312.
- Fan KH, et al: Chang Gung Med J 2009; 32: 526-534.
- Sugarbaker PH, Chang D: Ann Surg Oncol 1999; 6: 727-731.
- Chuanyu S, et al: Urology 2009; 74: 1036-1040.
InFo ONCOLOGY & HEMATOLOGY 2014; 2(8): 20-22.