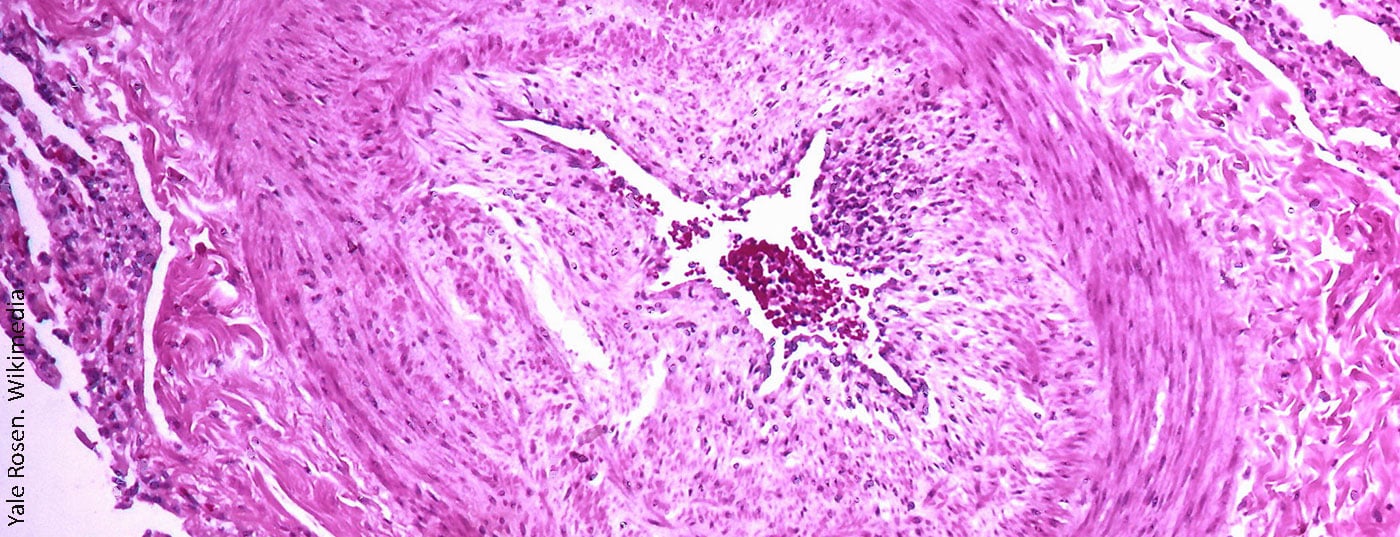
Chronic thromboembolic pulmonary hypertension (CTEPH) is a subtype of pulmonary hypertension (PH). The diagnosis is overall very rare, although CTEPH is also often unrecognized and thus underdiagnosed, and therefore the underreporting rate is probably higher.
Chronic thromboembolic pulmonary hypertension (CTEPH) is a subtype of pulmonary hypertension (PH), class 4 according to the ESC/ERC classification [1]. The diagnosis of CTEPH can be made when precapillary symptomatic PH (mPAP minimum 20-25 mmHg and pulmonary capillary occlusion pressure ≤15 mmHg) combined with perfusion disturbances in the lungs are present after a minimum of 3 months of reliable anticoagulation [2] for differentiation to acute pulmonary embolism (LE). The diagnosis is very rare overall, although CTEPH is also often unrecognized and thus underdiagnosed, and thus the underreporting rate is probably higher.
In this article, the diagnosis and therapy of CTEPH are presented with the aim of making general practitioners and also specialized colleagues more aware of this disease, because there is curative therapy for these patients, quite the opposite of all other forms of PH.
Incidence, risk factors and pathogenesis
The annual incidence for CTEPH in the United States, Europe, and Japan is approximately 3 to 5 cases per 100 000 population [2]. The cumulative incidence for CTEPH in the first 2 years after acute LE ranks between 0.1-9.1% [2–10], a wide range; for Switzerland, even one study reported only 0.79% [11]. To make matters worse, more than 25% of newly diagnosed cases do not have a history of acute LE [12]. There are other known risk factors besides thromboembolic events, such as autoimmune and hematologic disorders [13], the presence of ventriculo-atrial shunt or infected pacing electrodes, a history of splenectomy, a non-0 blood group, the presence of lupus anticoagulant/antiphospholipid antibodies, thyroid hormone replacement therapy, and a history of tumor [14], which are also prognostically unfavorable.
The exact pathophysiology of CTEPH is complex, remains unclear, and multiple causative mechanisms are likely present, such as persistent organized thrombi and scarring in the proximal pulmonary arteries (main, lobe, and segmental) and/or microangiopathy [15]. Various processes have been associated with incomplete thrombus resolution [16]: inflammatory [17–19], genetic factors [20–22], abnormalities of fibrinolysis [23–27] and many others [28].
Clinical picture and case description
The clinical picture always includes exertional dyspnea, the leading symptom [29,30]. Other symptoms include fatigue in about 30% of cases, chest pain in 15%, an episode of syncope in about 14%, and hemoptysis in 5% [12,31,32]. These symptoms are all not uncommon, especially in older patients with additional comorbidities such as chronic obstructive pulmonary disease, deconditioning, and often associated obesity [33,34]. To make matters worse, approximately half of all patients with acute LE report persistent shortness of breath, even years after an event, thus termed “post-PE syndrome.” Despite this, these patients should be further diagnostically worked up to rule out CTEPH.
Clinical examination reveals progressive edema of the lower extremities and other signs of congestion (neck veins, liver enlargement) [33]. Furthermore, auscultation may reveal an accentuation of the pulmonary component of the second heart sound.
Diagnosis
Unfortunately, to date, there is no biomarker that clearly differentiates CTEPH from other forms of PH; some circulating inflammatory markers (for example, CRP, interleukins -6, -8, and -10) have been studied [17,18,35], but none of these tests has routine use to date. Signs of right ventricular strain can be seen on the ECG, such as right-type, (in)complete right bundle branch block or T-wave negativities in anterior leads V1-V4 (“right ventricular strain pattern”). On chest x-ray, there are only nonspecific signs of right heart strain with an enlarged right ventricle and atrium, for example.
In further diagnostics, transthoracic echocardiography (TTE) is usually used first, which is also listed in the consensus conference of 2016 as the first diagnostic measure in the diagnostic algorithm (Fig. 1). It also represents a good initial screening test in differentiation from post-PE syndrome. Pulmonary hypertension is defined in TTE by the expression of tricuspid regurgitation velocity [36], from which sPAP can be measured. On Doppler-derived measurement of regurgitation velocity (Vmax) across the tricuspid valve, the simplified Bernoulli equation (∆P=4×Vmax2) is used to estimate the pressure gradient between the right ventricle and the right atrium. Right atrial pressure is usually approximated by inferior vena cava width and respiratory variability. Other signs include size and dilation of the right ventricle and atria, “D-shaping” of the septum, and tricuspid plane motion (“tricupsid annulat plane systlic excursion,” TAPSE). However, a normal echo does not rule out CTEPH.
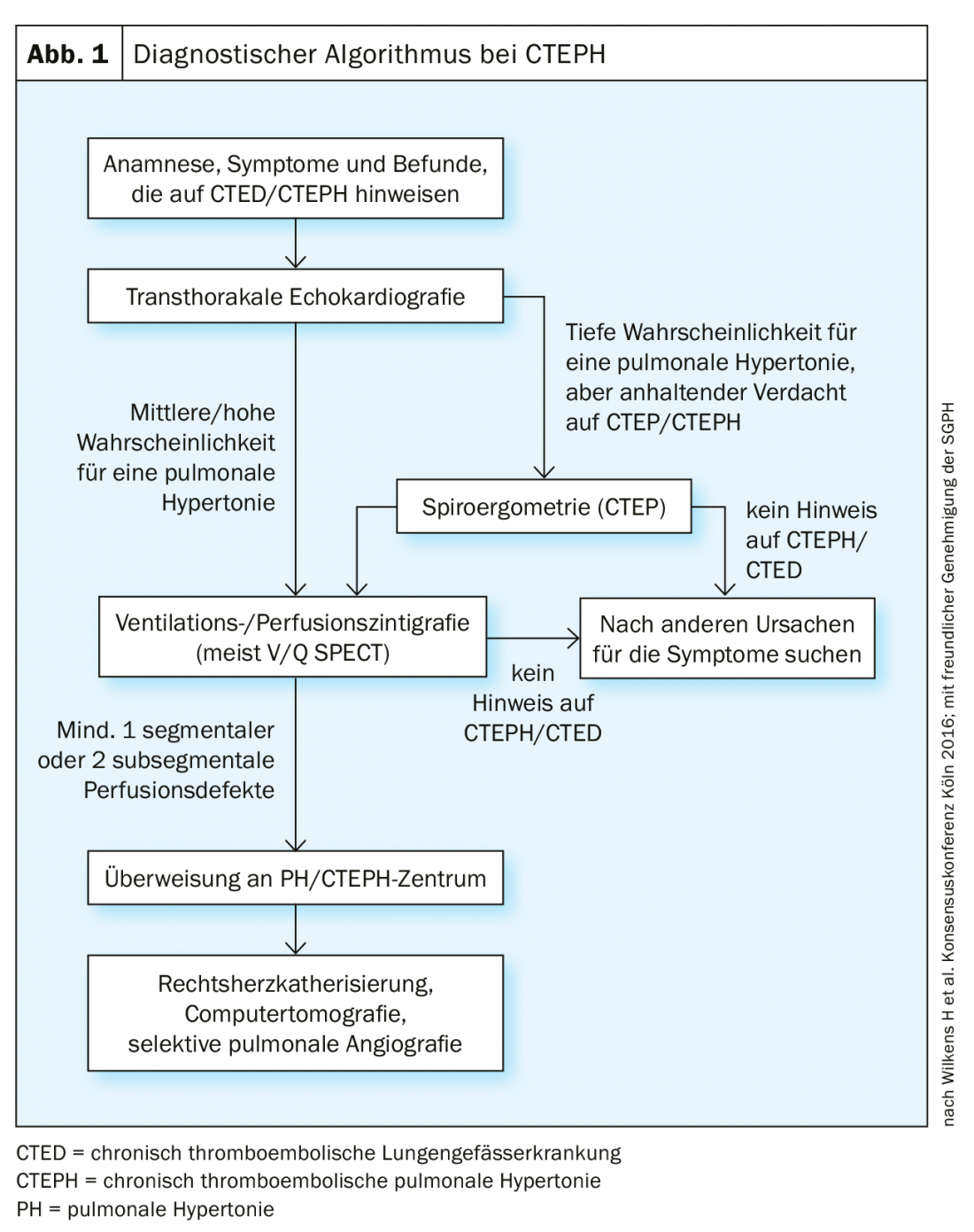
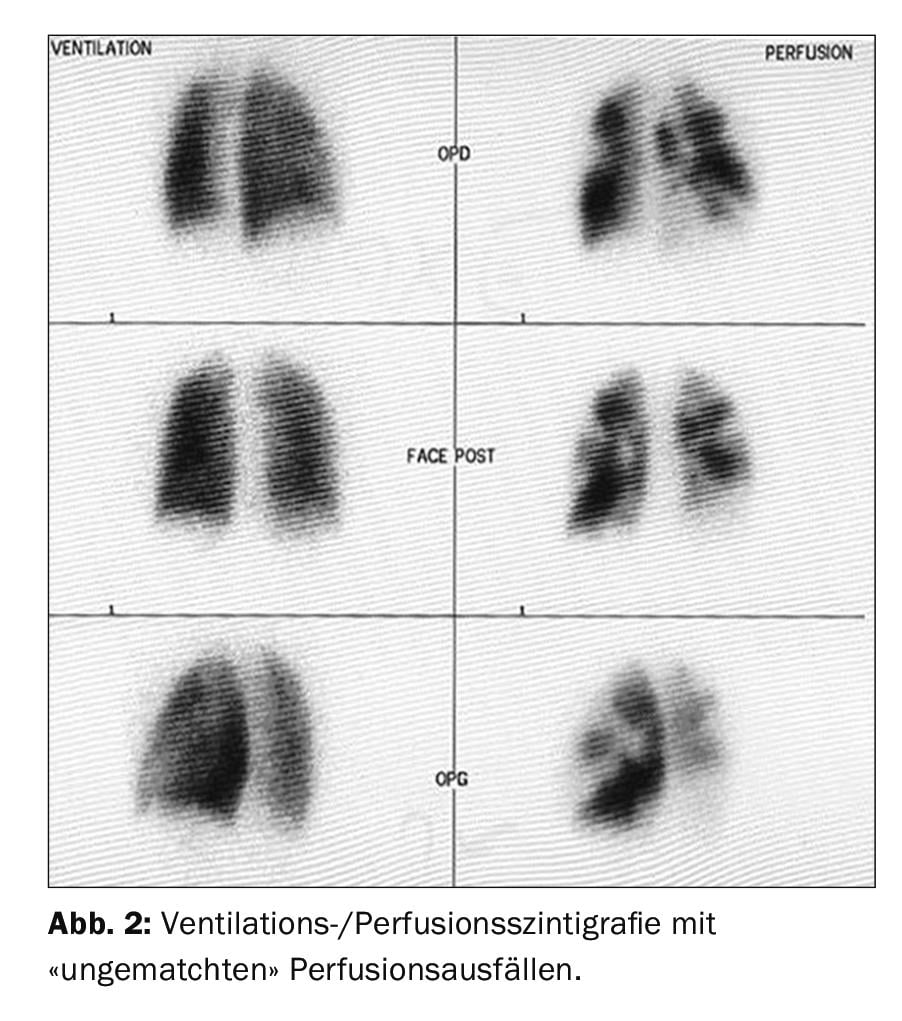
The first imaging screening method is represented by ventilation/perfusion scintigraphy (Fig. 2), which, if normal (i.e., without “unmatched” perfusion deficits) one can exclude CTEPH with a sensitivity of 90-100% and a specificity of 94-100% [37,38]. New “dual-energy” techniques with a hybrid combination of SPECT and CT have the advantage of simultaneously imaging the lung parenchyma in addition to perfusion deficits [39,40], but are not available in every hospital.
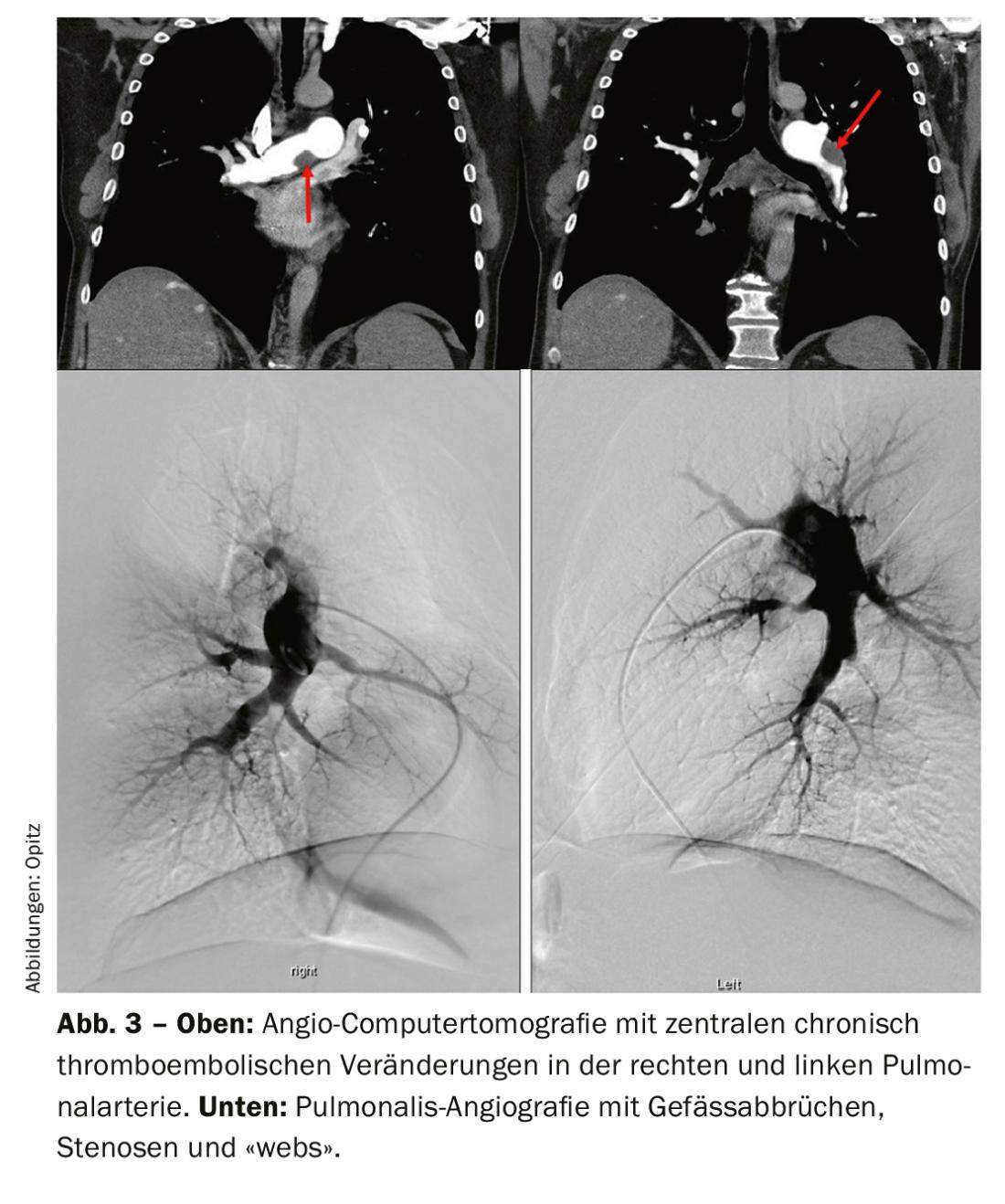
Computed tomographic pulmonary angiography (CTPA) has many advantages in the diagnosis and also assessment of operability for CTEPH. In particular, it has sensitivity and specificity for detecting thromboembolic changes at the lobar level (97-100% and 95-100%, respectively), and somewhat lower at the more peripheral segmental level (86-100 and 93-99%, respectively) [33,41–43]. The classic signs are eccentric thrombi (Fig. 3 above), so-called “webs,” and occluding “pouch-lesions,” as well as prominent collaterals from the intercostal arteries, for example. These signs are all also evident on pulmonary angiography (Fig. 3 bottom), which also has the advantage of invasive PA pressure measurement. However, other differential diagnoses (DD) can be detected on CT, and one also detects signs of “mosaic perfusion” in the lung parenchyma as well as infarcts, if any. Another advantage is the representation of the accessibility of chronic thromboembolic changes in identifying how proximal a dissection layer presents and whether it is accessible for pulmonary endarterectomy. To interpret these, reconstructions in sagittal and also coronal planes are needed in addition to the axial slice, and the CT should have been run with a slice thickness of 1 mm. Magnetic resonance imaging (MRI) has not yet played a role in CTEPH diagnosis in a standardized application; however, the simultaneous assessment of right ventricular function is an advantage, and in the future, MRI is expected to play an increasing role for screening, but also for follow-up of CTEPH patients after surgery or other therapy, as appropriate.
Hemodynamic diagnostic confirmation of CTEPH is made by right heart catheterization: Mean pulmonary arterial pressure (mPAP) should be ≥20-25 mmgHg [1] and precapillary (“wedge pressure”/pulmonary capillary occlusion pressure ≤15 mmHg). The acquisition of cardiac output by thermodilution or the direct Fick method is a prerequisite for a correct calculation of pulmonary vascular resistance (PVR), an important factor regarding prognosis and surgical risk [44].
Spiroergometry represents another functional examination in the delineation of CTEPH: Here, the typical signs of ventilation-perfusion mismatch with increased respiratory equivalents at the anaerobic threshold, an increased alveo-arterial pO2 gradient, positive capillary-end-tidal CO2 gradient, exercise hypoxemia, and clearly decreased exercise capacity are evident [45–48].
It is particularly important that if CTEPH is suspected, the patient should be referred to a specialized center for further interdisciplinary diagnosis and therapy allocation.
The following is a classic case with the entire patient history:
A 50-year-old patient with multiple pulmonary emboli and history of thromboembolic events presents with NYHA III-IV shortness of breath. In the 6-minute walk test, the patient reached 270 meters at Borg 2 (Table 1).
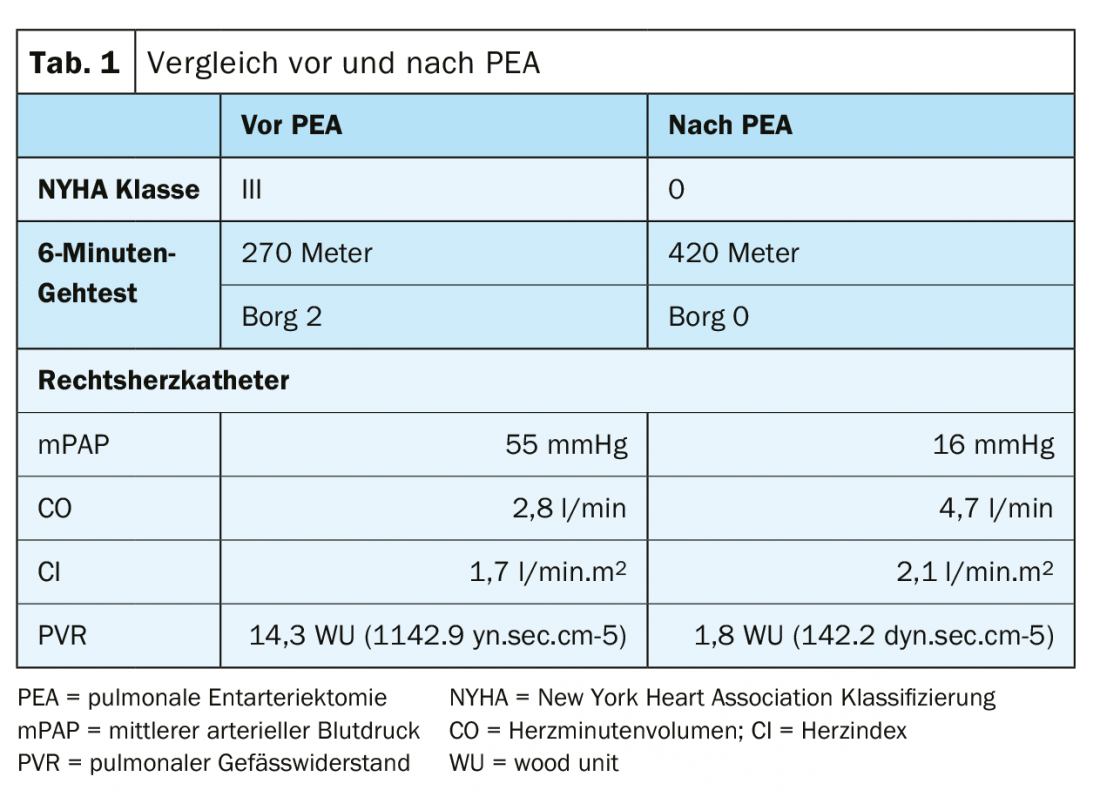
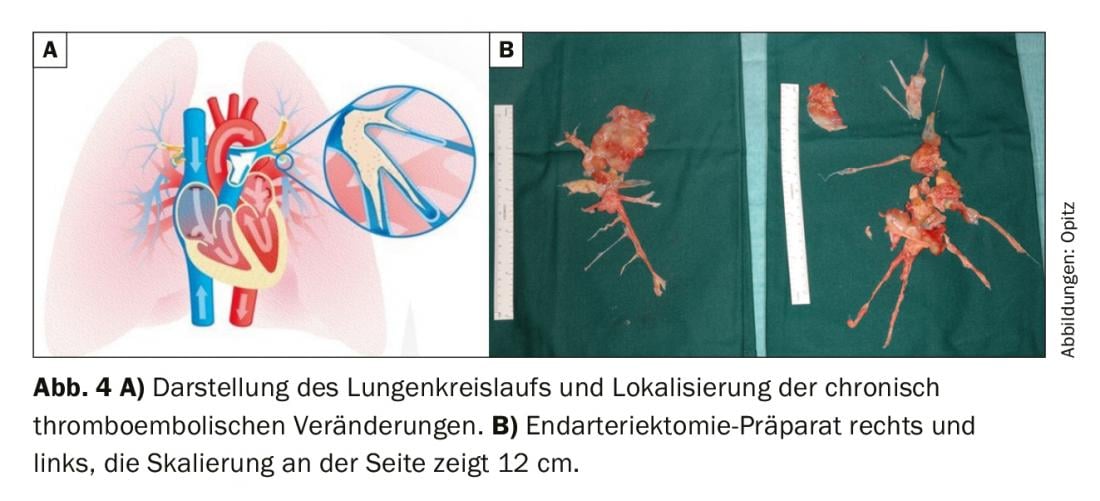
Ventilation/perfusion scintigraphy showed bilateral “unmatched” perfusion defects (Fig. 2) . Transthoracic echocardiography showed elevated systolic pulmonary artery pressure (sPAP) and dilated right ventricle. CT shows central lesions (fig. 3 , top), which were removed to the periphery during pulmonary entarterectomy (fig. 4B) . Postoperatively, the patient recovered rapidly and had normal hemodynamics and NYHA 0 at the 1-year follow-up (Table 1).
Forecast
Once the diagnosis is made, treatment should be discussed immediately because, if left untreated, a mPAP of >50 mmgHg has a 2-year mortality of >80% and a mPAP of >30 mmgHg is associated with a 3-year mortality of 90% [49].
Since 2015, an interdisciplinary CTEPH program has been offered at the University Hospital Zurich (USZ), providing the entire treatment spectrum from surgery to angioplasty and drug therapy. Once or twice a month, a national CTEPH board is offered through the Swiss Pulmonary Hypertension Society for referring physicians to contact for case presentation and discussion (CTEPH@usz.ch, CTEPH@sgph.ch).
Therapy
Surgical therapy: according to current ERS/ESC guidelines, pulmonary endarterectomy (PEA) is recommended as first-line therapy in patients with surgically accessible CTEPH [1]. (Fig. 4A). In PEA, the pulmonary arteries are sequentially opened (always bilaterally) on the heart-lung machine and in deep hypothermia (20°C), and the chronic thromboembolic material is removed in 20-minute circulatory arrests; this is technically possible well into the periphery (Fig. 4B). In any case, the decision for surgery and patient selection should be made by an interdisciplinary team of specialists, consisting of PH specialists, radiologists, and PEA surgeons [50]. The determining factors for PEA are: the severity of symptoms, the extent of PH, the function of the right ventricle, the patient’s comorbidity profile, and surgically accessible material. This last point in particular is a challenging assessment because it is well known that imaging almost always underestimates the extent of chronic thromboembolic material. Patients with segmental and subsegmental disease can also benefit from PEA with excellent short- and long-term outcomes in experienced centers [51,52]. Recommendations made during the World Symposium on PH state that a “second opinion” should be obtained from a surgical CTEPH expert center when a patient has been deemed inoperable at a non-CTEPH center [50]. Also, severity of PH and limitation of right heart dysfunction are not contraindications per se for PEA and symptomatic patients should be offered surgical therapy [53]. Age is also not an absolute contraindication, as data show that patients >70 years benefit as much as younger patients [54]. However, mortality from the procedure is not entirely insignificant and is reported in the literature to be 2.4-13.2%, the latter being from earlier periods [55].
Successful surgery can significantly improve patients’ quality of life and expectancy (survival rates of >90% after 1 year >70%, after 6 to 10 years [31,55,56]): Dyspnea, 6-minute walk test, and increased oxygen uptake, improved respiratory equivalent forCO2, and decreased need for oxygen were demonstrated [57,58]. Increased life expectancy has been reported in the medium to long term.
Pulmonary Balloon Angioplasty (BPA)
If surgery is not an option, interventional BPA can treat peripheral segmental or subsegmental chronic changes in multiple sessions. Convincing improvements in hemodynamics, 6-minute walk test, and NYHA/WHO class can be achieved. Currently, long-term data are still missing [59].
Drug therapy: patients with distal, nonsurgically accessible CTEPH and residual pulmonary hypertension after PEA can be treated with medication [50,60]. Drugs similar to those used in PAH are used [61–64]. Regardless of pressure-lowering therapy, which acts particularly on the distal component of CTEPH, every patient with CTEPH must be anticoagulated for life, regardless of other therapies. Therapeutic decisions should be made only after at least 3 months of OAC [1,65]. Currently, patients are still treated with vitamin K antagonists. Due to their comparable efficacy and good safety profile, NOAKs (new oral anticoagulants) are also increasingly used, especially also in cases of dose-finding problems and in cases where the INR (International Normalized Ratio) is often outside the target range of 2.5-3.5 [65,66].
Take-Home Messages
- Chronic thromboembolic pulmonary hypertension (CTEPH) is defined as symptomatic pulmonary hypertension with persistent pulmonary perfusion defects despite adequate anticoagulation for at least 3 months.
- CTEPH patients should be referred to a specialized CTEPH center for confirmation of the diagnosis by right heart catheterization and subsequent therapy.
- The Swiss Pulmonary Hypertension Society offers a national CTEPH board for referring physicians to contact for case presentation and discussion (CTEPH@usz.ch, CTEPH@sgph.ch).
- Patients’ functional capacity, exercise tolerance, and even life expectancy can be increased by pulmonary endarterectomy. For this reason, a PEA should be evaluated in every case.
- Drug therapy and/or balloon angioplasty may help patients with unresectable CTEPH or postoperative residual pulmonary hypertension.
- CTEPH mandatorily requires lifelong oral anticoagulation.
|
CTEPH team of the University Hospital Zurich: |
Literature:
- Galie N, Humbert M, Vachiery JL, et al: 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). The European respiratory journal. 2015;46(4): 903-975.
- Gall H, Hoeper MM, Richter MJ, et al: An epidemiological analysis of the burden of chronic thromboembolic pulmonary hypertension in the USA, Europe and Japan. European respiratory review : an official journal of the European Respiratory Society. 2017;26(143).
- Korkmaz A, Ozlu T, Ozsu S, et al: Long-term outcomes in acute pulmonary thromboembolism: the incidence of chronic thromboembolic pulmonary hypertension and associated risk factors. Clin Appl Thromb Hemost. 2012;18(3): 281-288.
- Otero R, Oribe M, Ballaz A, et al: Echocardiographic assessment of pulmonary arterial pressure in the follow-up of patients with pulmonary embolism. Thrombosis research. 2011;127(4): 303-308.
- Poli D, Grifoni E, Antonucci E, et al: Incidence of recurrent venous thromboembolism and of chronic thromboembolic pulmonary hypertension in patients after a first episode of pulmonary embolism. J Thromb Thrombolysis. 2010;30(3): 294-299.
- Surie S, Gibson NS, Gerdes VE, et al: Active search for chronic thromboembolic pulmonary hypertension does not appear indicated after acute pulmonary embolism. Thrombosis research. 2010;125(5): e202-205.
- Dentali F, Donadini M, Gianni M, et al: Incidence of chronic pulmonary hypertension in patients with previous pulmonary embolism. Thrombosis research. 2009;124(3): 256-258.
- Marti D, Gomez V, Escobar C, et al: Incidence of symptomatic and asymptomatic chronic thromboembolic pulmonary hypertension. Arch Bronconeumol. 2010;46(12): 628-633.
- Klok FA, van Kralingen KW, van Dijk AP, et al: Prospective cardiopulmonary screening program to detect chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism. Haematologica. 2010;95(6): 970-975.
- Noble S, Pasi J: Epidemiology and pathophysiology of cancer-associated thrombosis. Br J Cancer. 2010;102 Suppl 1: S2-9.
- Coquoz N, Weilenmann D, Stolz D, et al: Multicentre observational screening survey for the detection of CTEPH following pulmonary embolism. The European respiratory journal. 2018;51(4).
- Pepke-Zaba J, Delcroix M, Lang I, et al: Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation. 2011;124(18): 1973-1981.
- Blauwet LA, Edwards WD, Tazelaar HD, McGregor CG: Surgical pathology of pulmonary thromboendarterectomy: a study of 54 cases from 1990 to 2001. Hum Pathol. 2003;34(12): 1290-1298.
- Bonderman D, Wilkens H, Wakounig S, et al: Risk factors for chronic thromboembolic pulmonary hypertension. The European respiratory journal. 2009;33(2): 325-331.
- Kim NH: Group 4 Pulmonary Hypertension: Chronic Thromboembolic Pulmonary Hypertension: Epidemiology, Pathophysiology, and Treatment. Cardiol Clin. 2016;34(3): 435-441.
- Simonneau G, Torbicki A, Dorfmuller P, Kim N: The pathophysiology of chronic thromboembolic pulmonary hypertension. European respiratory review: an official journal of the European Respiratory Society. 2017; 26 (143).
- Quarck R, Nawrot T, Meyns B, Delcroix M: C-reactive protein: a new predictor of adverse outcome in pulmonary arterial hypertension. Journal of the American College of Cardiology. 2009;53(14): 1211-1218.
- Zabini D, Heinemann A, Foris V, et al: Comprehensive analysis of inflammatory markers in chronic thromboembolic pulmonary hypertension patients. The European respiratory journal. 2014;44(4): 951-962.
- Reesink HJ, Meijer RC, Lutter R, et al: Hemodynamic and clinical correlates of endothelin-1 in chronic thromboembolic pulmonary hypertension. Circ J. 2006;70(8): 1058-1063.
- Wolf M, Boyer-Neumann C, Parent F, et al: Thrombotic risk factors in pulmonary hypertension. The European respiratory journal. 2000;15(2): 395-399.
- Bonderman D, Turecek PL, Jakowitsch J, et al: High prevalence of elevated clotting factor VIII in chronic thromboembolic pulmonary hypertension. Thromb Haemost. 2003;90(3): 372-376.
- Gu S, Su P, Yan J, et al: Comparison of gene expression profiles and related pathways in chronic thromboembolic pulmonary hypertension. Int J Mol Med. 2014;33(2): 277-300.
- Lang IM, Dorfmuller P, Vonk Noordegraaf A: The Pathobiology of Chronic Thromboembolic Pulmonary Hypertension. Ann Am Thorac Soc. 2016;13 Suppl 3: S215-221.
- Le Gal G, Delahousse B, Lacut K, et al: Fibrinogen Aalpha-Thr312Ala and factor XIII-A Val34Leu polymorphisms in idiopathic venous thromboembolism. Thrombosis research. 2007;121(3): 333-338.
- Suntharalingam J, Goldsmith K, van Marion V, et al: Fibrinogen Aalpha Thr312Ala polymorphism is associated with chronic thromboembolic pulmonary hypertension. The European respiratory journal. 2008;31(4): 736-741.
- Marsh JJ, Chiles PG, Liang NC, Morris TA: Chronic thromboembolic pulmonary hypertension-associated dysfibrinogenemias exhibit disorganized fibrin structure. Thrombosis research. 2013;132(6): 729-734.
- Morris TA, Marsh JJ, Chiles PG, et al: High prevalence of dysfibrinogenemia among patients with chronic thromboembolic pulmonary hypertension. Blood. 2009;114(9): 1929-1936.
- Opitz I, Kirschner MB: Molecular Research in Chronic Thromboembolic Pulmonary Hypertension. Int J Mol Sci. 2019;20(3).
- Mayer E, Jenkins D, Lindner J, et al: Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg. 2011;141(3): 702-710.
- Held M, Grun M, Holl R, et al: Chronic thromboembolic pulmonary hypertension: Time delay from onset of symtoms to diagnosis and clinical condition at diagnosis. Dtsch Med Wochenschr. 2014;139(33): 1647-1652.
- Hoeper MM, Madani MM, Nakanishi N, et al: Chronic thromboembolic pulmonary hypertension. Lancet Respir Med. 2014;2(7): 573-582.
- Lang IM, Simonneau G, Pepke-Zaba JW, et al: Factors associated with diagnosis and operability of chronic thromboembolic pulmonary hypertension. A case-control study. Thromb Haemost. 2013;110(1): 83-91.
- Gopalan D, Delcroix M, Held M. Diagnosis of chronic thromboembolic pulmonary hypertension. European respiratory review: an official journal of the European Respiratory Society. 2017; 26 (143).
- Fedullo P, Kerr KM, Kim NH, Auger WR: Chronic thromboembolic pulmonary hypertension. American journal of respiratory and critical care medicine. 2011;183(12):1605–1613.
- Quarck R, Wynants M, Verbeken E, et al: Contribution of inflammation and impaired angiogenesis to the pathobiology of chronic thromboembolic pulmonary hypertension. The European respiratory journal. 2015;46(2): 431-443.
- Rehman MB, Garcia R, Christiaens L, et al: Power of resting echocardiographic measurements to classify pulmonary hypertension patients according to European society of cardiology exercise testing risk stratification cut-offs. International journal of cardiology. 2018;257: 291-297.
- Leblanc M, Leveillee F, Turcotte E: Prospective evaluation of the negative predictive value of V/Q SPECT using 99mTc-technegas. Nucl Med Commun. 2007;28(8): 667-672.
- Gruning T, Drake BE, Farrell SL, Nokes T. Three-year clinical experience with VQ SPECT for diagnosing pulmonary embolism: diagnostic performance. Clin Imaging. 2014;38(6): 831-835.
- Gutte H, Mortensen J, Jensen CV, et al: Detection of pulmonary embolism with combined ventilation-perfusion SPECT and low-dose CT: head-to-head comparison with multidetector CT angiography. J Nucl Med. 2009;50(12): 1987-1992.
- Simanek M, Koranda P: The benefit of personalized hybrid SPECT/CT pulmonary imaging. Am J Nucl Med Mol Imaging. 2016;6(4): 215-222.
- Ley S, Ley-Zaporozhan J, Pitton MB, et al: Diagnostic performance of state-of-the-art imaging techniques for morphological assessment of vascular abnormalities in patients with chronic thromboembolic pulmonary hypertension (CTEPH). Eur Radiol. 2012;22(3): 607-616.
- Reichelt A, Hoeper MM, Galanski M, Keberle M: Chronic thromboembolic pulmonary hypertension: evaluation with 64-detector row CT versus digital substraction angiography. Eur J Radiol. 2009;71(1): 49-54.
- Sugiura T, Tanabe N, Matsuura Y, et al: Role of 320-slice CT imaging in the diagnostic workup of patients with chronic thromboembolic pulmonary hypertension. Chest. 2013;143(4): 1070-1077.
- Jenkins D, Mayer E, Screaton N, Madani M: State-of-the-art chronic thromboembolic pulmonary hypertension diagnosis and management. European respiratory review: an official journal of the European Respiratory Society. 2012;21(123): 32-39.
- Scheidl SJ, Englisch C, Kovacs G, et al: Diagnosis of CTEPH versus IPAH using capillary to end-tidal carbon dioxide gradients. The European respiratory journal. 2012;39(1): 119-124.
- Held M, Meintz S, Baron S, et al: Surgical cure of central sleep apnea? American journal of respiratory and critical care medicine. 2013;188(3): 395-396.
- Held M, Grun M, Holl R, et al: Cardiopulmonary exercise testing to detect chronic thromboembolic pulmonary hypertension in patients with normal echocardiography. Respiration. 2014;87(5): 379-387.
- Held M, Linke M, Jany B: Echocardiography and right heart catheterization in pulmonary hypertension. Dtsch Med Wochenschr. 2014;139(30): 1511-1517.
- Lewczuk J, Piszko P, Jagas J, et al: Prognostic factors in medically treated patients with chronic pulmonary embolism. Chest. 2001;119(3): 818-823.
- Kim NH, Delcroix M, Jenkins DP, et al: Chronic thromboembolic pulmonary hypertension. Journal of the American College of Cardiology. 2013;62(25 Suppl): D92-99.
- Madani MM, Auger WR, Pretorius V, et al: Pulmonary endarterectomy: recent changes in a single institution’s experience of more than 2,700 patients. The Annals of thoracic surgery. 2012;94(1): 97-103; discussion.
- D’Armini AM, Morsolini M, Mattiucci G, et al: Pulmonary endarterectomy for distal chronic thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg. 2014;148(3): 1005-1011; 12 e1-2; discussion 11-2.
- Madani MM: Surgical Treatment of Chronic Thromboembolic Pulmonary Hypertension: Pulmonary Thromboendarterectomy. Methodist Debakey Cardiovasc J. 2016;12(4): 213-218.
- Berman M, Hardman G, Sharples L, et al: Pulmonary endarterectomy: outcomes in patients aged >70. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2012;41(6): e154-160.
- Cannon JE, Su L, Kiely DG, et al: Dynamic Risk Stratification of Patient Long-Term Outcome After Pulmonary Endarterectomy: Results From the United Kingdom National Cohort. Circulation. 2016;133(18): 1761-1771.
- Archibald CJ, Auger WR, Fedullo PF, et al: Long-term outcome after pulmonary thromboendarterectomy. American journal of respiratory and critical care medicine. 1999;160(2): 523-528.
- Rahnavardi M, Yan TD, Cao C, et al: Pulmonary thromboendarterectomy for chronic thromboembolic pulmonary hypertension: a systematic review. Annals of thoracic and cardiovascular surgery: official journal of the Association of Thoracic and Cardiovascular Surgeons of Asia. 2011;17(5): 435-445.
- Condliffe R, Kiely DG, Gibbs JS, et al: Improved outcomes in medically and surgically treated chronic thromboembolic pulmonary hypertension. American journal of respiratory and critical care medicine. 2008;177(10): 1122-1127.
- Mahmud E, Behnamfar O, Ang L, et al: Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Interv Cardiol Clin. 2018;7(1): 103-117.
- Mayer E: Surgical and post-operative treatment of chronic thromboembolic pulmonary hypertension. European respiratory review: an official journal of the European Respiratory Society. 2010;19(115): 64-67.
- Moser KM, Bloor CM: Pulmonary vascular lesions occurring in patients with chronic major vessel thromboembolic pulmonary hypertension. Chest. 1993;103(3): 685-692.
- Ghofrani HA, D’Armini AM, Grimminger F, et al: Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. The New England journal of medicine. 2013;369(4): 319-329.
- Simonneau G, D’Armini AM, Ghofrani HA, et al: Predictors of long-term outcomes in patients treated with riociguat for chronic thromboembolic pulmonary hypertension: data from the CHEST-2 open-label, randomised, long-term extension trial. Lancet Respir Med. 2016;4(5): 372-380.
- Ghofrani HA, Simonneau G, D’Armini AM, et al: Macitentan for the treatment of inoperable chronic thromboembolic pulmonary hypertension (MERIT-1): results from the multicentre, phase 2, randomised, double-blind, placebo-controlled study. Lancet Respir Med. 2017;5(10): 785-794.
- Konstantinides SV, Torbicki A, Agnelli G, et al: 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43): 3033-3069, 69a-69k.
- Konstantinides SV, Torbicki A, Agnelli G, et al: Corrigendum to: 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2015;36(39): 2642.
InFo PNEUMOLOGY & ALLERGOLOGY 2020; 2(2): 12-15.