Aneurysmal subarachnoid hemorrhage (aSAB) is associated with high mortality and morbidity. After initial stabilization of the patient, neurosurgical and/or endovascular therapies must be used to eliminate the source of bleeding. Aneurysms that are amenable to both therapeutic options should preferably be treated endovascularly. Nevertheless, not every aneurysm is suitable for endovascular therapy, and surgical options have a clear place to date. Nevertheless, due to the minimally invasive nature and the progressive advancement of the endovascular method, an increase in this form of therapy is expected. The purpose of this paper is to present the possibilities of both therapeutic options.
The sudden onset of an “annihilation headache” as the leading pathognomonic symptom of an aneurysmal subarachnoid hemorrhage (aSAB) due to rupture of a brain base aneurysm marks the beginning of a drastic event for most patients, which will at best shape the weeks to come, but often the rest of their lives [1]. This often affects young (mean age of onset 40-60 years) and otherwise healthy women and men (sex ratio 1.6:1). The clinical presentation of aSAB can vary widely but often includes severe headache, meningismus, nausea/vomiting, and reduced vigilance. At best, the constellation of symptoms leads to rapid hospitalization and diagnosis by computed tomography (CT) and, if necessary, cerebrospinal fluid (CSF) puncture. If the suspected diagnosis is made and confirmed only after a delay, this can have a detrimental effect on further therapy and the clinical-functional outcome, which is why a high level of attention is always required when assessing patients with headache.
Management of the aSAB
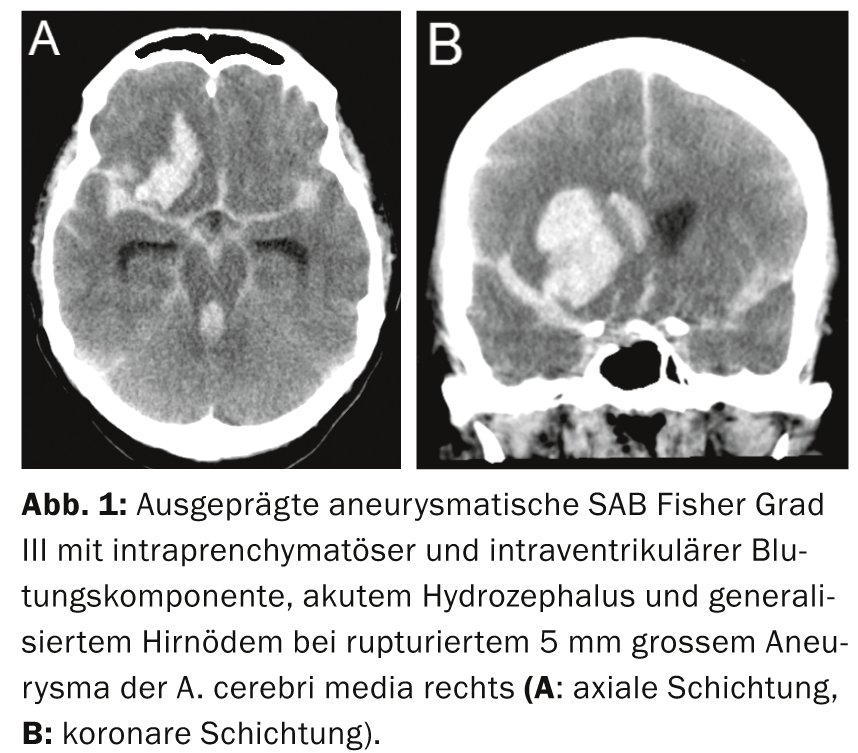
Diagnosis and classification: When a patient with suspected SAB is referred, after vital signs are secured and an orienting neurologic examination is performed (Table 1), the diagnosis should be quickly confirmed by CT (Fig. 1a and b) , and SAB can be classified according to the Fisher scale (Table 2) [2].
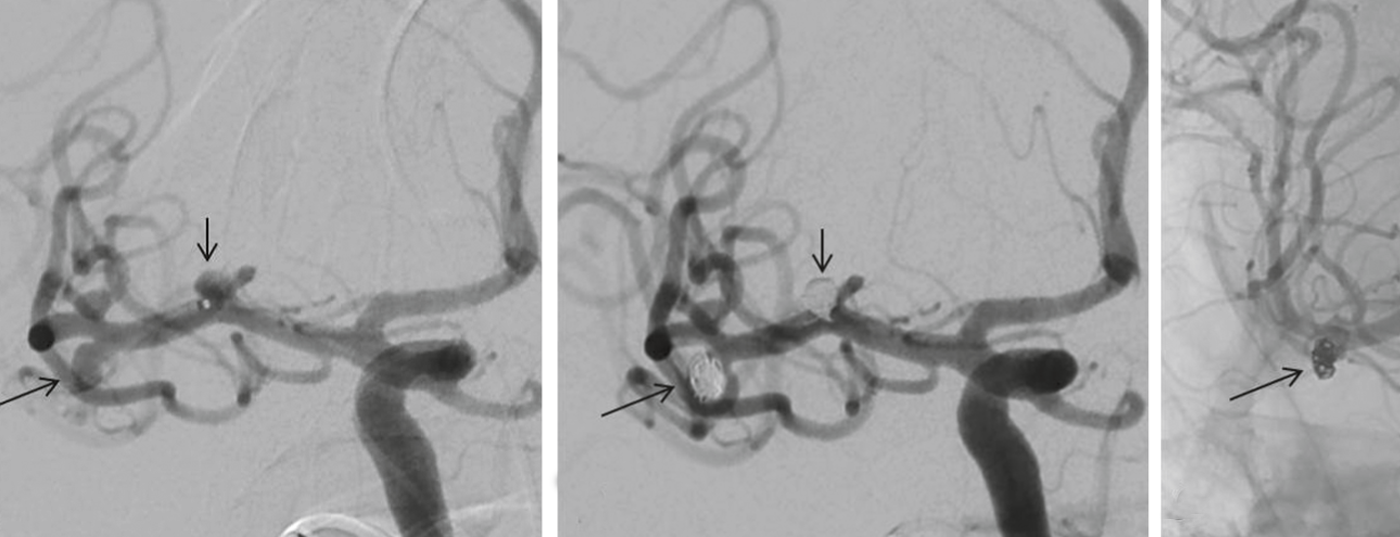
Magnetic resonance imaging may be helpful if there is a prolonged interval between bleeding events and initial diagnosis; CT is preferable in acute cases. In the absence of evidence of hemorrhage on CT, CSF puncture to detect erythrocytes (and possibly siderophages/xantochromia) is essential if clinical suspicion is high. If SAB is present, angiography should be sought to differentiate aneurysmal from nonaneurysmal SAB and to identify the source of bleeding. CT angiography (CT-A) can be performed in the same session as native CT, and modern CT-A can reliably diagnose the source of bleeding in many cases. However, it only serves as a basis for decision-making regarding subsequent occlusion therapy in patients with an acute life-threatening condition requiring immediate surgical intervention (e.g., space-occupying extraaxial or intracerebral hemorrhage). The best visualization of the bleeding-symptomatic aneurysm (and the morphology of the aneurysm neck), additional asymptomatic aneurysms if any, and the patient-specific vascular anatomy is achieved with conventional 4-vessel angiography.
Perioperative management: The preoperative goal is to prevent postoperative hemorrhage and to treat symptomatic intracranial hypertension. In patients with aSAB and reduced vigilance, look for the presence of acute hydrocephalus and relieve it by inserting an external ventricular drain (EVD). Subsequent monitoring is indicated in a specialized (neuro)surgical intensive care unit. Until the aneurysm is definitively eliminated, postoperative bleeding may occur (risk about 4% in 24 h and 20% in 2 weeks), which is why blood pressure should be controlled and blood pressure peaks avoided. Patients after aSAB may experience a number of acute complications (including electrolyte imbalances, epileptic seizures, pulmonary and cardiac complications), the detailed management of which is beyond the scope of this paper and has been described in detail elsewhere [3].
In the postoperative period, prophylaxis, diagnosis, and therapy of disease-specific complications associated with an increased risk of secondary cerebral infarction are crucial. Cerebral vasospasm (CVS) occurs in 30-70% of patients from the second day after aSAB, reaches its maximum between the sixth and ninth day, and becomes symptomatic during this period in approximately 50% of cases (DCI, “delayed cerebral ischemia”). DCI is the most common cause of secondary cerebral infarction, morbidity, and mortality after aSAB. The calcium antagonist nimodipine reduces the risk of death or need for long-term care (“relative risk” 0.67; 95%CI 0.55-0.81), so it should be administered prophylactically. If medical hemodynamic-augmentative measures are insufficient to treat symptomatic CVS, endovascular treatment by balloon angioplasty and/or superselective instillation of vasodilators (papaverine, nimodipine) may be necessary in selected cases.
Occlusion therapy
Aneurysm occlusion serves as prophylaxis against rebleeding and should be attempted as soon as possible, but in any case within 72 hours after the bleeding event [4]. There are basically two methods for this: microsurgical or endovascular intervention.
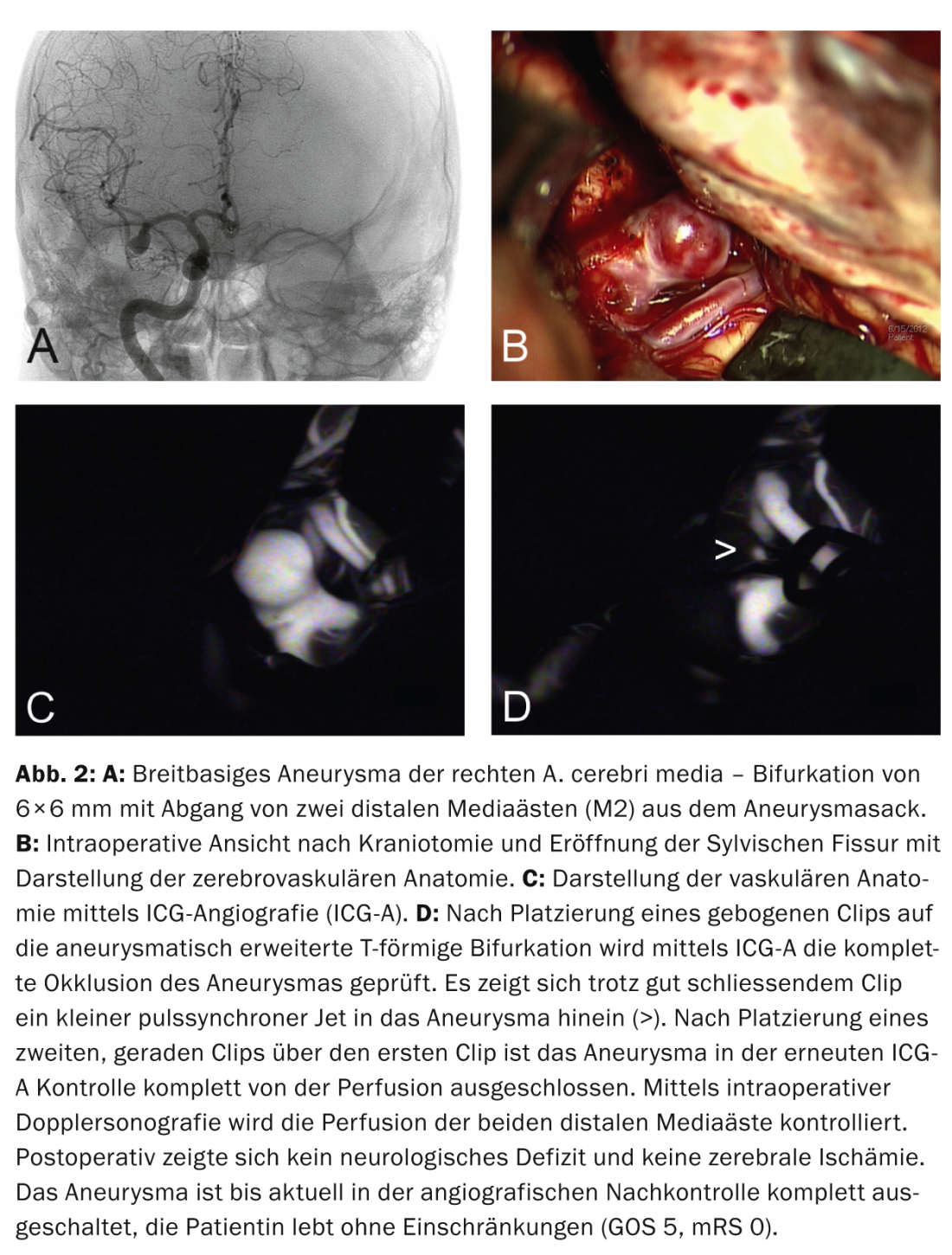
Aneurysm clipping and other surgical therapies: Microsurgical treatment of intracranial aneurysms by clipping requires opening of the cranial dome and meninges, visualization of the cerebrovascular anatomy (Fig. 2a and b), and placement of a clip on the aneurysm neck. Intraoperative fluorescein angiography (ICG angiography) can be used to check for complete aneurysm occlusion (Fig. 2c and d); intraoperative Doppler sonography can detect accidental occlusion of adjacent vessels, requiring clip repositioning. Other microsurgical treatment alternatives include wrapping and trapping of the aneurysm.
Wrapping involves surrounding the aneurysm sac with tissue (muscle, fascia) or non-absorbable foreign tissue. This method is used in fusiform vasodilatations or in aneurysms from whose sac important vessels arise. Thus, definitive elimination is not always possible, and the protective effect is controversial.
In trapping (usually for fusiform or giant aneurysms), the carrier vessel is occluded using clips proximal and distal to the aneurysm. If the collateral supply to the supply area is insufficient, extra-intracranial bypass surgery must follow. Detailed preoperative assessment of cerebral perfusion and function is essential to avoid irreversible brain damage.
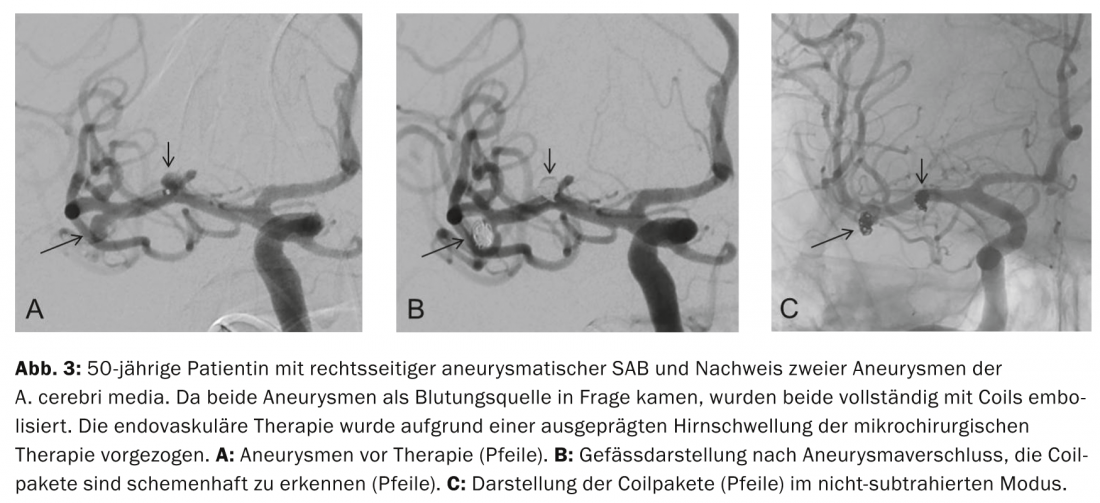
Aneurysm coiling and other endovascular procedures: The principle of endovascular aneurysm coiling, introduced by Guglielmi in 1991, is based on thrombosis induced in the aneurysm by electrolytically deposited coils after platinum coils have been filled into the aneurysm sac via a catheter advanced endovascularly into the aneurysm. Complete closure of the aneurysm sac with coils prevents recurrence of aneurysm rupture (Fig. 3a to c) .
A new and promising therapeutic approach is balloon-assisted coiling and the combination of stent implantation and coiling to improve the occlusion rate in wide neck aneurysms. In recent years, endovascular therapy options have been expanded by the development of flow-diverters and new implantation materials (e.g. WEB).
Selection of the optimal therapeutic option: the optimal method for occlusion of the ruptured aneurysm should always be selected on an interdisciplinary basis by vascular neurosurgeons and interventional neuroradiologists [4], with the locally available expertise playing a major role in the selection of the therapeutic modality. Nowadays, there is evidence, among others from the ISAT trial (“International subarachnoid aneurysm trial”), that endovascular treatment (coiling without balloon or stent) leads to a better clinical-functional outcome after one year compared to clipping [5]. In the ISAT trial, 2142/9559 patients with aneurysms that could have been treated by either method were randomized to a treatment arm (in 77.6% of the patients evaluated, one method was a priori preferable-they were excluded from the study). In addition to a high selection bias, the ISAT study was criticized for the disproportionately large percentage (90%) of mild forms of SAB and an underrepresentation of aneurysms of the middle cerebral artery. However, for the included subpopulation, coiling (23.7%) was shown to result in an absolute risk reduction of 6.9% for death and severe morbidity one year after therapy compared with clipping (30.6%). While longer follow-up of ISAT patients also showed a reduction in 5-year relative mortality in favor of coiling, the rate of independently living patients was the same after clipping (82%) and coiling (83%) [6]. Both initial and subsequent ISAT data showed an increased rate of rebleeding in coiled patients, including a high rate of incomplete aneurysm occlusions [5, 6]. As a result, 17.4% of patients who were coiled and 3.8% of those who were clipped underwent retreatment (hazard ratio = 6.9) [7], casting a critical light on the long-term benefit from coiling alone, particularly in younger patients <40 years [8]. However, future comparative studies allowing stent-assisted and/or balloon-assisted endovascular treatment are expected to yield better results regarding long-term aneurysm occlusion after endovascular therapy. It remains unclear what the difference is that led to a better outcome in patients after coiling in ISAT. Discussed is the higher invasiveness of craniotomy with more postoperative complications (“systemic inflammatory response syndrome”, anemia, etc.) and a lower incidence of CVS and DCI after coiling therapy.
In a recent meta-analysis, both methods were analyzed with respect to secondary endpoints [9]. The risk of CVS was found to be reduced in endovascularly treated patients (n=1267; 43.1% vs. 48.8%; OR 1.43; 95%CI 1.07-1.91; p=0.02), with similar rates of ischemic infarction after endovascular or microsurgical therapy (n=1123; 20.9% vs. 16.1%; OR 0.74; 95%CI 0.52-1.06; p=0.10). Similar numbers of patients required a definitive shunt (n=1981; 19.3% vs. 16.4%; OR 0.84; 95%CI 0.66-1.07; p=0.16), and the rate of periprocedural complications was comparable between the two methods (n=866; 5.6% vs. 9.9%; OR 1.19; 95%CI 0.67-2.11; p=0.56) [9]. What is clear is that the ISAT results cannot be applied to the entire patient population with aSAB and that both forms of therapy will continue to coexist, as in certain situations one method is clearly preferable to the other. Nevertheless, the ISAT results challenge neurovascular surgeons to continue to refine their treatment and to select patients who are better served with surgical therapy in the future. In the following, the decision-making process for one or the other therapy option will be outlined.
Preferred endovascular care: Endovascular treatment is generally preferred in elderly patients (>70 years), patients at high medical surgical risk, or with poor neurological status (WFNS grade 4 and 5) because of its less invasiveness [4]. Furthermore, aneurysms in the posterior fossa (vertebrobasilar stromal area) or the cavernous sinus should preferably be treated endovascularly, since surgical treatment in these localizations is associated with significantly increased morbidity and mortality and is sometimes not feasible at all. Narrow neck aneurysms are particularly suitable for endovascular treatment – should a wide aneurysm neck require the combination of coiling and stent placement, the rate of periprocedural complications is somewhat increased. Clipping may be preferable, also in view of the need to take aspirin and clopidogrel after stenting, which compromises the possibility of neurosurgical treatment (EVD, craniectomy).
Furthermore, coiling is preferable in patients on medication to thin the blood (e.g., aspirin, clopidogrel, Marcoumar) and in the presence of multiple bilateral aneurysms not accessible via a surgical approach. In patients who require care within the vasospasm phase, endovascular therapy may be preferred because surgical dissection of the base of the brain is significantly more difficult when the hemisphere is swollen (“angry brain”) and any manipulation of the vessels by the surgeon during this vulnerable phase may result in CVS. In contrast, angiographically visible vasospasm during endovascular treatment can be effectively treated by immediate angioplasty or local intra-arterial application of vasodilators. Supported by the ISAT data, coiling should be preferred in cases where both treatment options appear equally effective [4].
Preferred neurosurgical care: Surgical therapy is preferred in emergency situations when additional space-occupying extraaxial or intracerebral hemorrhage requires immediate surgical hematoma evacuation (Fig. 1). Clipping can be performed in the same session if (CT) angiographic aneurysm visualization is of sufficient quality beforehand. Furthermore, surgical therapy is recommended for patients with aneurysms in the area of the A. cerebri media or A. pericallosa (in case of a narrow neck, however, endovascular treatment is also good), in case of aneurysms with a wide neck or outgoing vessels from the aneurysm sac (danger of occlusion in case of coiling; Fig. 2) or in aneurysms not readily accessible by endovascular means (e.g., marked “kinking” of the internal carotid artery, patients with fibromuscular dysplasia and risk of iatrogenic carotid dissection) is recommended; in younger patients <40 years of age, it is worth considering [4, 8]. In the presence of multiple unilateral aneurysms, multiple aneurysms can be accessed through the same approach during one surgical procedure.
Outcome and outlook
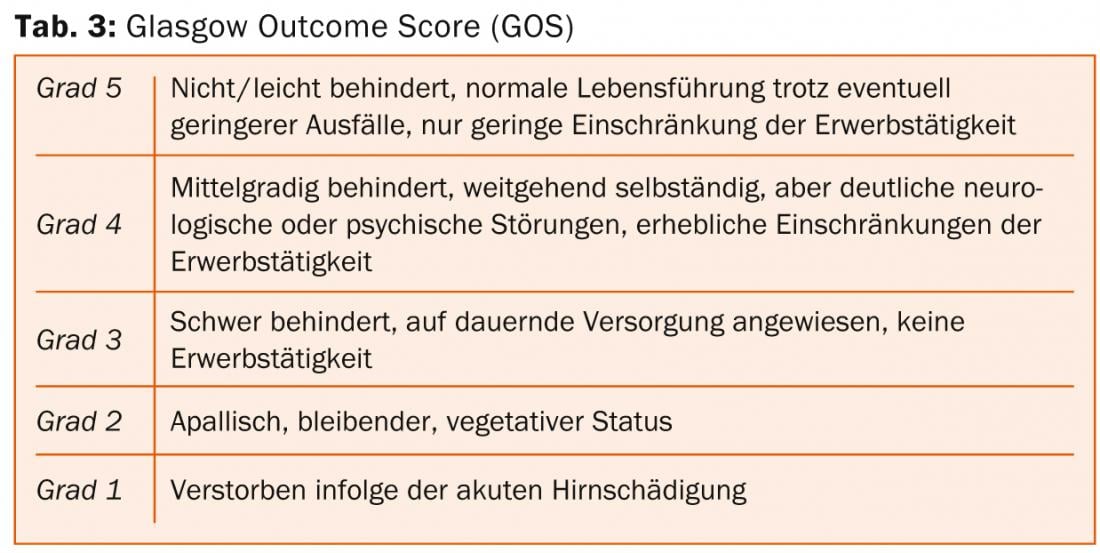
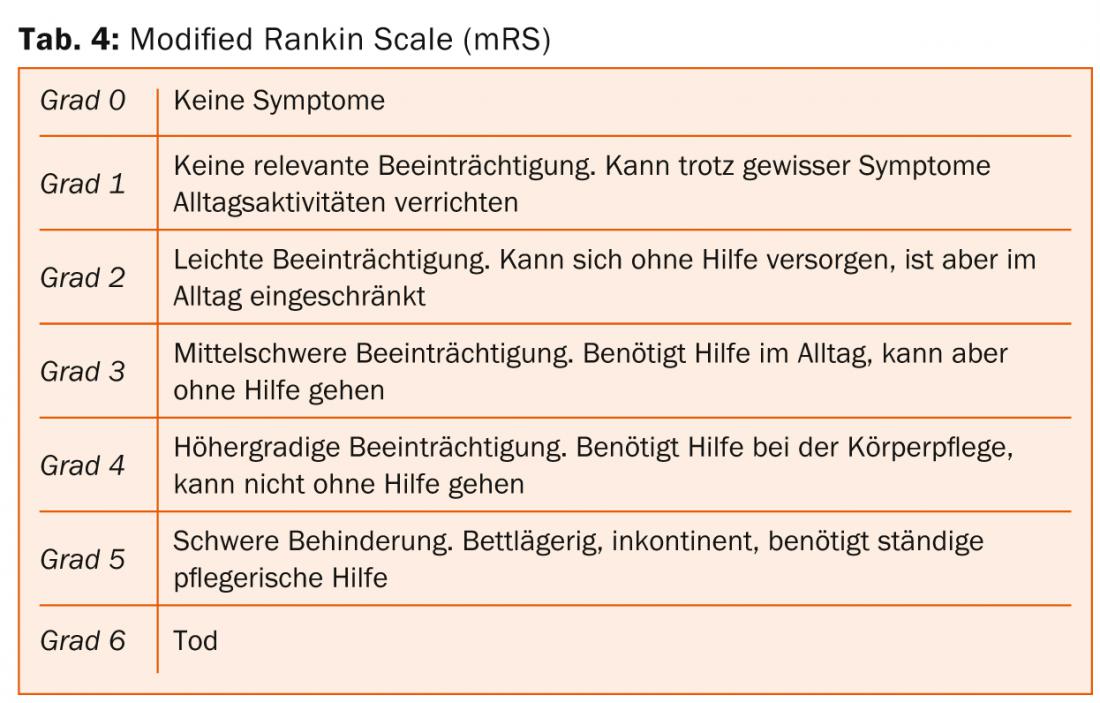
The clinico-functional outcome after aSAB depends mainly on age and severity of the initial hemorrhage. In recent decades, mortality after aSAB has been reduced from about 50% in the 1970s to about 30% currently. Nowadays, most patients die before hospital admission. The percentage of patients with severe SAB and good clinical-functional outcome (“Glasgow Outcome Scale” [GOS] 4-5 [Tab. 3] or “modified Rankin Scale” [mRS] 0-2 [Tab. 4]) is about 40% today, but the incidence and everyday relevance of neuropsychological deficits after aSAB is high even in patients with optimal neurological outcome [1].
The development regarding the future selection of the optimal occlusion therapy depends significantly on further technical advances and the long-term results of large comparative studies. At the Cantonal Hospital St.Gallen, a consensus is first reached between neurosurgery and neuroradiology regarding the optimal form of therapy for each aSAB patient, after which the patient and relatives are jointly informed.
In line with the trend in all areas of surgery toward minimally invasive, less traumatic, and more cosmetically benign methods, the proportion of patients treated by endovascular means is likely to continue to increase in the future. Other, more recent aspects of aneurysm management are clipping as well as coiling of residual aneurysms (after incomplete coiling/clipping, coil compaction or increase in size of the aneurysm despite coiling/clipping), which may pose new challenges to the therapist.
Global developments are also followed with great interest within Switzerland, and insufficiently clarified issues are addressed. In this country, the big eight neurovascular centers have joined forces to document the care of aSAB patients, to collect crucial parameters in a national database (“Swiss study on aneurysmal subarachnoid haemorrhage”, Swiss SOS) and thus to contribute to a better understanding and prognosis of this complex disease. [10].
Martin N. Stienen, MD
Acknowledgments: The authors thank Dr. Heiko Richter, OAmbF Neurosurgery (KSSG) for kindly providing Figure 2.
Literature:
- Stienen MN, et al: Acta Neurochir Vienna 2013; 155: 2045-2051.
- Fisher CM, Kistler JP, Davis JM: Neurosurgery 1980; 6: 1-9.
- Seule MA, et al: Anasthesiol Intensivmed Notfallmed Schmerzther 2010; 45: 8-17.
- Steiner T, et al: Cerebrovasc Dis 2013; 35: 93-112.
- Molyneux A, et al: Lancet 2002; 360: 1267-1274.
- Molyneux AJ, et al: Lancet Neurol 2009; 8: 427-433.
- Campi A, et al: Stroke 2007; 38: 1538-1544.
- Mitchell P, et al: J Neurosurg 2008; 108: 437-442.
- Li H, et al: Stroke 2013; 44: 29-37.
- Schatlo B, et al: Acta Neurochir Vienna 2012; 154: 2173-2178; discussion 2178.
InFo Neurology & Psychiatry 2014; 12(1): 18-22.