Migraine is the most common neurological disorder and severely affects the quality of life of those affected. To prevent chronicity, prophylactic measures are becoming increasingly important in migraine therapy. The principle of the new Calcitonin Gene-Related Peptide (CGRP) antibodies is to start where the pain begins.
Primary headache disorder, characterized by 4-72-hour-lasting, moderate-to-severe, unilateral, pulsating-throbbing headache attacks typically accompanied by accompanying vegetative symptoms, is a serious neurological disorder. Yet it is still often underdiagnosed, as Prof. Messoud Ashina, MD, Copenhagen, cautioned at a satellite symposium initiated by TEVA at the 5th Congress of the European Academy of Neurology (EAN). However, the symptoms, such as headache attacks, nausea, sensitivity to light and sound, place a heavy burden on those affected and not infrequently lead to significant impairments in daily life. If the symptoms occur on at least 15 days/month, of which they meet the diagnostic criteria for migraine on at least eight days, the migraine is chronic. To prevent this process, patients should receive adequate prophylaxis in addition to effective acute therapy.
Until now, the main drugs available for preventive treatment have been beta-blockers, calcium channel blockers, and anticonvulsants. However, since these were not specifically developed for migraine prophylaxis, they entail a broad spectrum of side effects. Therefore, many patients discontinue oral prophylaxis after a short time. Accordingly, intensive research has been conducted into new, targeted, effective and well-tolerated substances. A key role in the development of migraine attacks is played by calcitonin gene-related peptide (CGRP), which induces vasodilation and increases cerebral blood flow, reported Prof. Anthony Dickenson, MD, London. During an attack, CGRP levels are elevated in the brain and meninges. Against this background, the CGRP antibodies were developed, which are now considered a possible new milestone in migraine prophylaxis due to the positive study data.
Migraine management under control
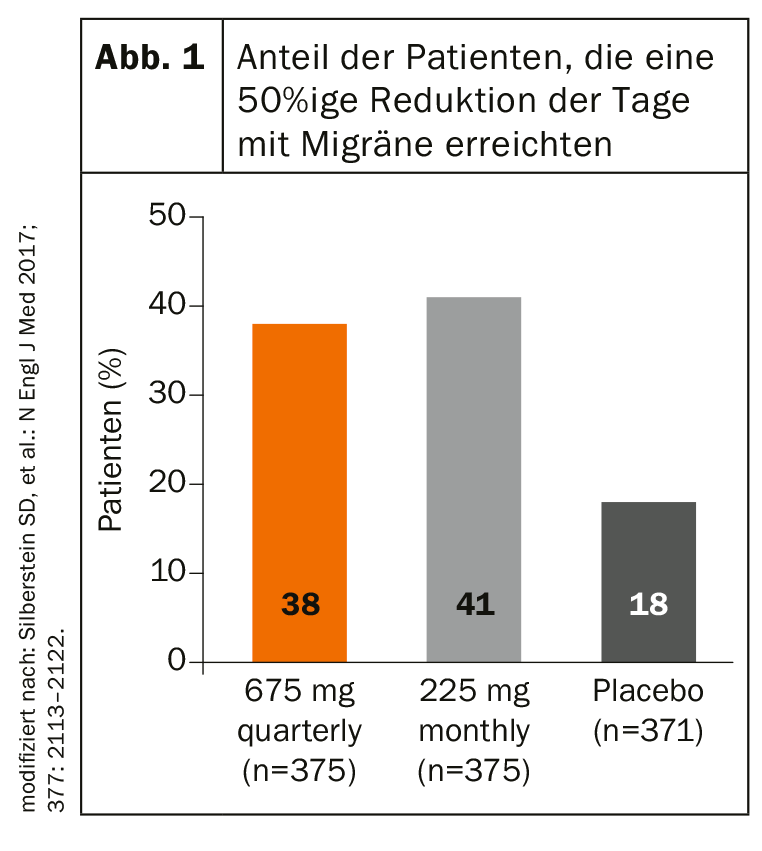
With fremanezumab, erenumab and galcanezumab, there are three antibodies representing the new therapeutic option. While erenumab blocks the CGRP receptor, fremanezumab and galcanezumab bind directly to the neuropeptide. In phase III studies, fremanezumab has demonstrated its remarkably early onset of action in both episodic and chronic migraine. As early as week four, there was a significant difference compared to placebo. Patients with episodic migraine were treated with fremanezumab injection or placebo once or three times at four-week intervals during the three-month study. In the verum groups, the mean number of monthly headache days decreased significantly more, 2.9 days (subcutaneous injection per quarter) and 3.0 days (monthly subcutaneous injection), compared with 1.6 days for placebo. In the chronic migraine study, the number of monthly days with migraine decreased by an average of 4.3 and 4.6 in the verum group, respectively (placebo: -2.5 days). Also, the proportion of patients with at least a 50% reduction in monthly days with migraine was significantly higher under fremanezumab (Fig. 1) . These effects also remained stable in the long term over a period of 12 weeks, as Prof. Zaza Katsarava, MD, Unna, Germany, explained.
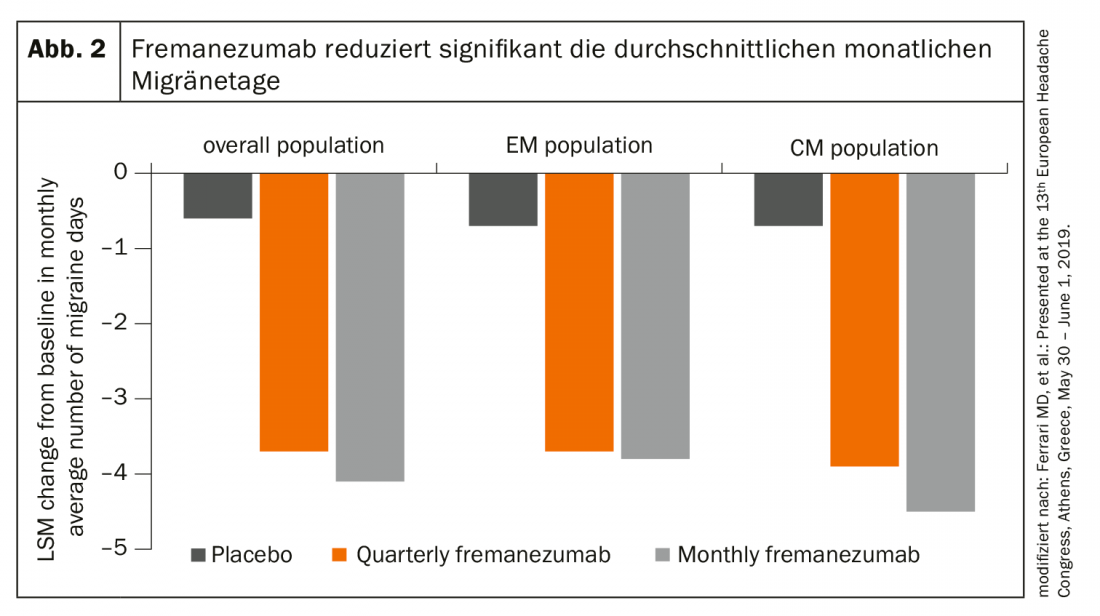
In addition, a Focus study demonstrated that the CGRP antibody significantly reduces both the average number of migraine days and the use of acute migraine medication after just one week of treatment – in episodic and chronic migraine (Fig. 2). Good safety and tolerance were also demonstrated in the long term.
Source: TEVA
InFo NEUROLOGY & PSYCHIATRY 2019; 17(5): 32.











