In the context of prevalent type 2 diabetes (T2D) worldwide and associated comorbid cardiovascular disease, the results of the SUSTAIN-6 trial add to the accumulating evidence of robust cardiovascular risk reduction with glucagon like-peptide-1 receptor agonist (GLP-1RAs) treatment.
GLP-1RAs with proven cardiovascular (CV) benefits are recommended in patients with type 2 diabetes and atherosclerotic CV disease (ASCVD) or at high risk for it [2,3]. This was driven in part by the SUSTAIN-6 study evaluating cardiovascular and other long-term outcomes with semaglutide in patients with T2D, a randomized, double-blind, placebo-controlled study to evaluate the cardiovascular safety of once-weekly subcutaneously administered semaglutide at two doses (0.5 and 1.0 mg) compared with placebo (volume-matched doses) in 3297 patients with T2D with or at high risk for cardiovascular disease [4]. In analyses in which doses were pooled as prospectively planned, semaglutide reduced the risk of the primary composite end point of cardiovascular death, nonfatal myocardial infarction (MI), or nonfatal stroke, as well as the exploratory end point of coronary or peripheral revascularization [4]. In a recently published study, SUSTAIN-6 data were used to investigate the effect of semaglutide compared with placebo on composite coronary artery disease-specific endpoints in exploratory post-hoc analyses [1].
Pooled post hoc analyses of semaglutide versus placebo.
Patients included in the SUSTAIN-6 trial had T2D, and to the extent that they were ≥50 years of age, had prevalent cardiovascular disease (defined as prior cardiovascular, cerebrovascular, or peripheral vascular disease), chronic heart failure, or chronic kidney disease. If age ≥60 years, patients had to have ≥1 CV risk factor, such as persistent microalbuminuria or proteinuria, hypertension and left ventricular hypertrophy, left ventricular systolic or diastolic dysfunction, or an ankle-brachial index <0.9. Patients were randomized to receive 0.5 or 1.0 mg semaglutide subcutaneously once weekly or placebo, with or without background glucose-lowering therapy. In the post hoc analyses, all patients who received semaglutide were pooled and compared with all patients who received placebo, as was done in the prospectively planned analysis of the primary outcome. The main coronary end point was a composite of time to first fatal/nonfatal myocardial infarction or coronary revascularization, defined as coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI). The extended coronary outcome was a composite of the main coronary outcome or time to first hospitalization for unstable angina. Subgroup analyses were performed for patients with and without prior myocardial infarction and/or coronary revascularization at baseline. Time to first event during the study was analyzed with the stratified Cox proportional hazard model. The Ghosh-Lin estimator was used for secondary analyses of time to first and recurrent events during the study, with all-cause death considered as a competing risk [5]. The percentage of patients with first events and the event rate for first and recurrent events at the end of the study are also reported. P values less than 0.05 were considered significant. No adjustments were made for multiplicity.
A total of 3297 patients were included in the present analyses: 1648 in the semaglutide group and 1649 in the placebo group (the same population as in the original analyses) [4]. The median follow-up time was 2.1 years [4]. For the post hoc analyses, the main cohort characteristics included a mean age of 64.6 ± 7.4 years and a T2D duration of 13.9 ± 8.1 years; 39.3% were women and 48.3% had a previous myocardial infarction or coronary revascularization. In the semaglutide and placebo groups, 781 and 812 patients, respectively, had suffered a previous myocardial infarction or coronary revascularization at baseline. Semaglutide reduced the risk of first major coronary event compared with placebo with a hazard ratio (HR) of 0.74 (95% confidence interval [CI] 0.57 to 0.97); the Number Needed to Treat (NNT) was 52 (Fig. 1) [1]. Semaglutide also reduced the risk of first extended coronary event compared with placebo with a HR of 0.73 (95% CI 0.56 to 0.94); NNT 46. Prior myocardial infarction or coronary revascularization at baseline did not change the treatment effect for either end point (all P interactions >0.05). The total number of major coronary events (including first and repeat events), as assessed by the Ghosh-Lin estimator, was significantly lower with semaglutide compared with placebo (4.5 vs. 6.0 events per 100 years of observation; mean ratio, 0. 74 [95% CI 0,55 bis 0,99]) and a similar effect, although not significant, was observed for the extended coronary outcome (5.2 vs. 6.8 events per 100 years of observation; mean ratio 0.75 [95% CI 0,56 bis 1,00]).
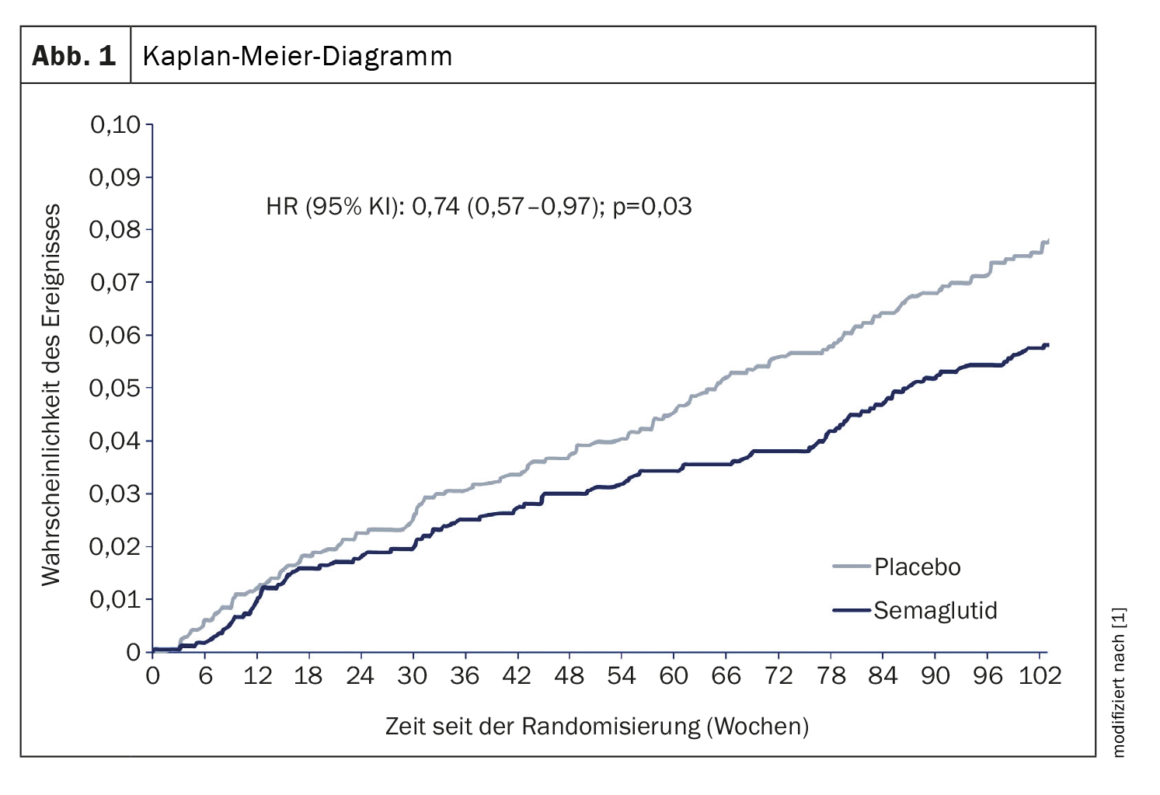
Risk reduction of first events
In the exploratory post-hoc analyses of the SUSTAIN-6 trial, once-weekly subcutaneous semaglutide reduced the risk of the first event included in the main and extended coronary outcomes compared with placebo in patients with T2D with or at high risk of cardiovascular disease; similar results were observed in the analysis of first and recurrent events. This decrease was mainly due to a reduction in coronary revascularization events. Results for first events were consistent in subgroups with or without prior myocardial infarction or coronary revascularization at baseline. These results extend previous observations regarding the CV benefit of semaglutide in patients at high risk for ASCVD complications, supporting the indication for the product for the prevention of cardiovascular death, myocardial infarction, and stroke in patients with T2D and cardiovascular disease [6]. The results also support the broad support for priority use in the Society’s recommendations and guidelines [2,3,7,8]. Complementing the evidence on reduction in incident coronary events, the impact on overall events of primary and extended coronary end points reinforces the benefit by showing a broader reduction in recurrent events, which represent a significant burden of morbidity in these patients [4].
Literature:
- Kolkailah AA, et al: Effects of once-weekly subcutaneous semaglutide on coronary artery disease outcomes in patients with type 2 diabetes with or at high risk for cardiovascular disease: Insights from the SUSTAIN-6 trial. Diabetes Obes Metab 2022; doi: https://doi.org/10.1111/dom.14941.
- Draznin B, Aroda VR, Bakris G, et al: 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2022. Diabetes Care. 2022; 45(Suppl 1): S125-s143.
- Cosentino F, Grant PJ, Aboyans V, et al: 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020; 41(2): 255-323.
- Marso SP, Bain SC, Consoli A, et al: Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016; 375(19): 1834-1844.
- Furberg JK, Rasmussen S, Andersen PK, Ravn H: Methodological challenges in the analysis of recurrent events for randomised controlled trials with application to cardiovascular events in LEADER. Pharm Stat. 2022; 21(1): 241-267.
- Novo Nordisk. Ozempic® (semaglutide) Prescribing Information. 2022; www.accessdata.fda.gov/drugsatfda_docs/label/2022/209637Orig1s009lbl.pdf. Accessed August 2022.
- Joseph JJ, Deedwania P, Acharya T, et al: Comprehensive Management of Cardiovascular Risk Factors for adults with type 2 diabetes: a scientific statement from the American Heart Association. Circulation 2022; 145(9): e722-e759.
- Joseph JJ, Deedwania P, Acharya T, et al: Comprehensive management of cardiovascular risk factors for adults with type 2 diabetes: a scientific statement from the American Heart Association. Circulation 2022; 145(9): e722–e759.
CARDIOVASC 2023; 22(1): 24–25