Stopping the progression to manifest dementia is one of the overarching therapeutic goals in the treatment of mild cognitive impairment (MCI). A randomized placebo-controlled trial investigated related effects of two traditional Chinese medicine medicines. In both MCI patients treated with Qinggongshoutao and Ginkgo biloba, the proportion of patients who developed AD over the course of one year was significantly lower than with placebo.
Mild cognitive impairment (MCI) is defined as a transitional state between normal aging processes and the early phase of dementia and, according to the S3 guideline, can be determined on the basis of the clinical picture and with the inclusion of neuropsychological testing procedures [1]. Affected individuals suffer from memory impairment, but everyday functions are not or only slightly impaired [2]. MCI is a risk condition for the development of dementia and may be a prodromal stage of Alzheimer’s type dementia (“AD”). In about 5-10% of cases, MCI progresses to Alzheimer’s disease or another form of dementia; in others, cognitive abilities remain stable for a long time or improve again [3]. Vascular, metabolic, toxic, and psychological risk factors are an important therapeutic target. Amnestic variant of MCI (“amnestic mild cognitive impairment”, aMCI) is considered to be the most common prodromal syndrome of Alzheimer-type dementia (AD) [4].
Traditional Chinese herbal medicine as a treatment option
For the symptomatic treatment of neuropsychiatric symptoms in cases of mild cognitive impairment or to halt the progression to dementia, medicines from traditional Chinese medicine (TCM) are used, among others. In addition to the special ginkgo extract EGb 761®**, Qinggongshoutao (QGST#), a herbal pill based on a traditional Chinese recipe, is also used. QGST has antioxidant, neuroprotective and memory enhancing effects according to experimental studies and is used in TCM to treat forgetfulness, back pain, knee pain and incontinence [5,6]. EGb 761® is recommended in some guidelines as a treatment option for MCI [1]. Tebokan® 80/120/240 is a preparation containing EGb 761® that is approved in Switzerland for the symptomatic treatment of brain-organic mental performance deficits in dementia-related syndromes [8]. A randomized double-blind study by Tian et al. addressed the question of whether QGST can delay the clinical manifestation of AD in patients with aMCI compared with ginkgo extract (EGb761®) and placebo, respectively [5].
** Ginkgo extract (Ginkgo biloba, Ginkgoaceae) EGb 761 (Dr Willmar Schwabe GmbH & Co. KG; Karlsruhe, Germany)
# QGST (Tianjin Zhongxin Pharmaceutical Group Co., Ltd. Darentang Pharmaceutical Factory, branch number: 141001; China)
How effective are ginkgo biloba and QGST in placebo comparison?
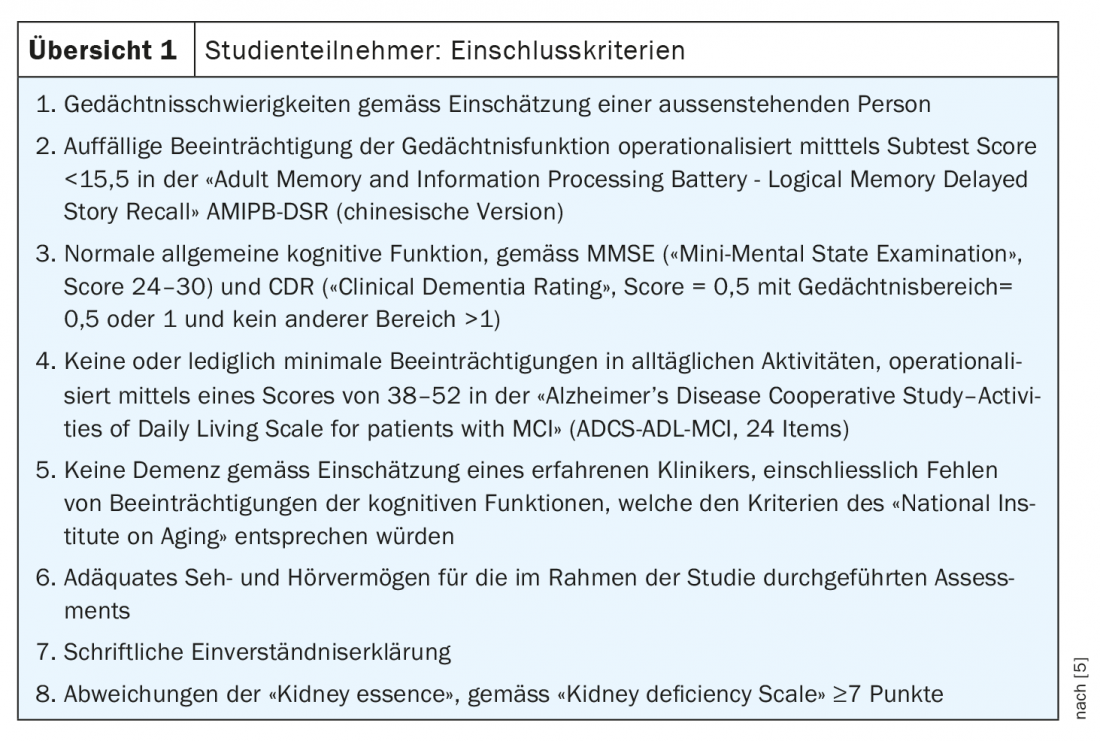
350 patients with mild cognitive impairment (Overview 1) were randomized to study participation at one of 17 implementation sites in China during 2015-2017 and, after a 2-week placebo “run-in” phase, received either 7 g QGST 2×/d (n=174) or 80 mg ginkgo extract 2×/d plus QGST placebo 2×/d (n=104) or QGST placebo 2×/d plus ginkgo placebo 2×/d (n=70).
The primary endpoints were, first, the proportion of patients who met criteria for AD at the end of the study (week 52) (as defined by the National Institute on Aging – Alzheimer’s Association Workgroups and Clinical Dementia Rating – Global Score (CDR-GS) ≥1) [7]. And second, the change in the cognitive subscale of the Alzheimer’s disease assessment scale-cognitive subscale (ADAS-cog) after twelve months. Secondary endpoints included changes from baseline to week 52 in the following scores: MMSE (“Mini-Mental State Examination”), “Delayed Story Recall” of the “Adult Memory and Information Processing Battery” (AMIPB-DSR), “Alzheimer’s disease cooperative study activities of daily living inventory for Alzheimer’s disease scale” (ADCS-ADL).
Conversion rate to Alzheimer’s dementia lower than with placebo
At 52 weeks after baseline, a total of 10 of all study participants met criteria for AD. In the QGST group, 1.15% of subjects were affected (n=2), in the ginkgo group, 0.96% (n=1), and in the placebo condition, 10% (n=7). Verum and placebo differed significantly, whereas QGST and Ginkgo biloba did not.
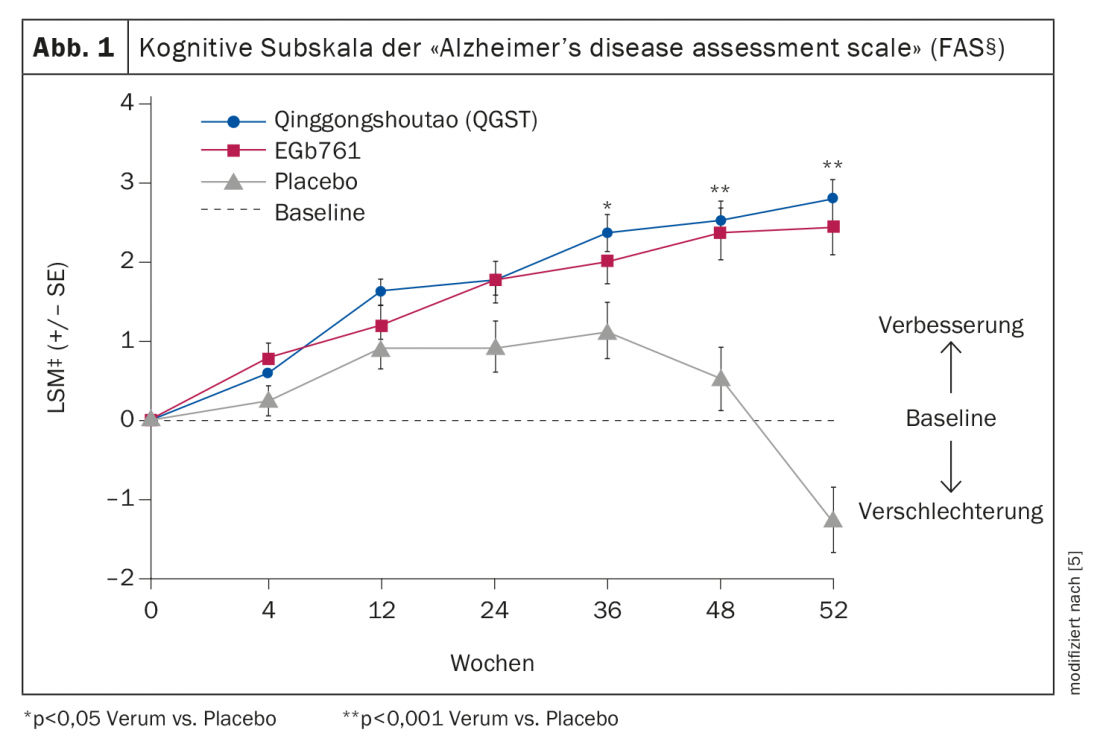
Those treated with QGST or Ginkgo biloba showed better results in cerebral function tests compared with placebo. Regarding the cognitive subscale of the Alzheimer’s disease assessment scale-cognitive subscale (ADAS-cog), the criterion for responders was defined as a change in score ≥-4. The responder rate at the end of the study was 29.27% under QGST and 27.84% under Ginkgo biloba, respectively, significantly higher in both treatment groups compared to placebo (p<0.001). Whereby the observation over time shows that the differences between placebo and verum reached significance level only after week 36. The least-square means (LSM) in the full-analysis set (FAS)* of ADAS-cog scores 52 weeks after baseline were 2.76 under QGST (n=174), 2.43 under Ginkgo biloba (n=104), and -1.25 (n=70) in the placebo arm, which also corresponds to a significant difference between verum and placebo (p<0.001) (Fig. 1) . And also regarding the changes in the MMSE (“Mini-Mental State Examination”), the LSM‡ differences verum vs. placebo 52 weeks after baseline proved to be significant (QGST = -0.92, Ginkgo = -0.86, placebo = 0.25), but those of the two treatment groups not.
‡ LSM = mean values for which the influence of covariates on the dependent variable was removed.
* FAS = Full Analysis Set: FAS included patients who had received at least one dose of the respective treatment regimen and for whom, in addition to the baseline values, the outcome parameters of at least one survey time point after randomization were available.
Treatment generally well tolerated
Differences in the proportion of subjects with at least one adverse event during the course of the study did not differ significantly between the three study arms (p=0.315) and were 50% in the QGST group (n=87), 41.35% in the ginkgo group (n=43), and 42.86% in the placebo condition (n=30). Of 348 study participants included in the full analysis set, a total of 16 discontinued treatment before week 52. In the QGST group, there was 1 drop-out due to adverse events; the other cases were for other reasons. Constipation occurred in 2 of the patients treated with QGST, and 1 discontinued the study because of this. The most commonly reported side effect with Ginkgo biloba was diarrhea. Of the 174 patients treated with QGST, 1.1% (n=2) had ≥1 serious adverse event, and among the 104 patients in the ginkgo group, this proportion was 3.8% (n=4). However, there was no dropout because of this.
Conclusion
The proportion of study participants who met criteria for Alzheimer’s disease (AD) after one year was significantly lower in MCI patients treated with QGST and Ginkgo biloba, respectively, than in the placebo condition. The conversion rates reported in the placebo group are consistent with data reported in the literature. According to the results in MMSE and ADAS-cog, cognitive functions improved under QGST and Ginkgo biloba, respectively, after 12 months of treatment. These results support the hypothesis that these herbal preparations help improve overall cognitive function in patients with amnestic MCI and reduce the risk of transition to AD. That QGST and ginkgo extract are safe and well-tolerated drugs was confirmed. As a limitation of the study, the authors mention that apolipoprotein Eε4 (ApoE ε4), a risk factor for progression from aMCI to AD, had not been collected. It was possible that the percentage of ApoE ε4 carriers was higher among study participants in the placebo group. Another limitation concerns the relatively short study duration over a period of 52 weeks, so that the question of the long-term efficacy of QGST or ginkgo extract cannot be answered in the present study.
Literature:
- DGPPN/DGN: S3-Leitlinie “Demenzen”, 2016, long version. www.dgppn.de
- McDade EM, Petersen RC: Mild cognitive impairment: epidemiology, pathology, and clinical assessment. UpToDate 10/2015.
- Huber F, Beise U: Dementia. Last revised: 03/2017. www.medix.ch/wissen/guidelines/psychische-krankheiten/demenz (last call 05/28/2021)
- Tondo G, et al:Alzheimer’s Disease Neuroimaging Initiative. Biomarker-based stability in limbic-predominant amnestic mild cognitive impairment. Eur J Neurol 2021; 28(4): 1123-1133.
- Tian J, et al: Chinese herbal medicine Qinggongshoutao for the treatment of amnestic mild cognitive impairment : a 52-week randomized controlled trial. Alzheimers & Dementia, Translational Research and Clinical Interventions 2019; 5: 441-449.
- Chen K, et al: Study on the anti-aging function of Qinggongshoutao pills. J Traditional Chin Med 1985: 25-28.
- McKhann GM, et al: The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011; 7: 263-269.
- Swissmedic: Medicinal product information, www.swissmedicinfo.ch, last accessed 28.05.2021
HAUSARZT PRAXIS 2021; 16(6): 28-29
InFo NEUROLOGY & PSYCHIATRY 2021; 19(6): 35.