What should be clarified in case of abnormal TSH values? Is there subclinical or manifest thyroid dysfunction? In which cases is an antibody determination or scintigraphy necessary? These and other questions related to thyroid disease were addressed at the FOMF Internal Medicine.
In the presence of symptoms and clinical evidence suggestive of thyroid disease, the following diagnostic options are available: (a) Laboratory diagnostics (TSH, fT4, and possibly fT3), (b) Sonography (primarily to detect nodules in the presence of struma), (c) Antibody determination (TRAK, TPO, TAK), (d) Fine needle aspiration (for nodules >2 cm), (e) Scintigraphy (for clarification of thyroid nodules in unclear hyperthyroidism) [1].
If a subclinical form of hypothyroidism is detected (TSH too high, fT3 and fT4 in the normal range), for example during a routine examination, follow-up is essential. The risk of developing manifest hypothyroidism increases when antibody testing is positive [2]. The same applies with regard to subclinical hyperthyroidism.

Subclinical and manifest hypothyroidism: what to watch out for?
Subclinical or latent hypothyroidism does not cause any symptoms, since sufficient thyroid hormones are still produced and only the TSH level is elevated (currently valid normal range: TSH <5 mU/l). The typical symptoms of manifest hypothyroidism (TSH too high, fT3 and fT4 too low) include bradycardia, hypertension, hyporeflexia, and hypothermia. Changes in the voice (deep, hoarse) and skin (pale yellowish) are also common. That hypothyroid coma occurs is very rare, Prof. Lehmann said. In terms of etiology, the most common is autoimmune thyroiditis (41% of cases) [3], a chronic inflammatory disease of the thyroid gland, which is referred to as Hashimoto’s syndrome or chronic lymphocytic thyroiditis. In the early stages of this disease, hyperthyroidism occurs for a short time (hashitoxicosis), and in the later course, hypothyroidism develops. Diagnostic markers include lymphocytic infiltrates and the presence of antibodies to a thyroid enzyme (TPO positive in 80-99%, TAK positive in 35-60%) [3], as well as a diffuse goiter in the presence of the hypertrophic form of Hashimotos’ syndrome (as distinguished from the atrophic form). The second most common cause is idiopathic; in about 37% of cases, no cause is detectable [3]. Third most common are posttherapeutic hypothyroidism, e.g., after radiation or after total/subtotal thyroidectomy. Nowadays, congenital hypothyroidism (cretinism) is only rare (about 9% of cases); in Switzerland, all newborns are screened for this. Drug-induced hypothyroidism is present in approximately 2.5% of cases [3]. Active substances and substance classes that can lead to hypothyroidism are thyrostatic drugs (iodization inhibitors), amiodarone (arrhythmic drug), lithium (psychopharmaceutical), interferon-α, interleukin-2, tyrosine kinase inhibitors (used in chemotherapy; e.g. sunitinib and sorafenib).
The standard therapy of hypothyroidism is hormone replacement (thyroxine, fT4) according to the following dosing scheme: start with expected maintenance dose: 1.6 µg/kg body weight 30 minutes before breakfast. A dose reduction should be made in elderly patients (>60 years) and individuals with coronary artery disease. Once the target values are reached, a dose adjustment should also be made as part of therapy progress monitoring; fT4 target value after two weeks: 14-16 nmol/l; TSH target value in the long-term course (after 6 weeks at the earliest): 0.5-2 mU/l [3].
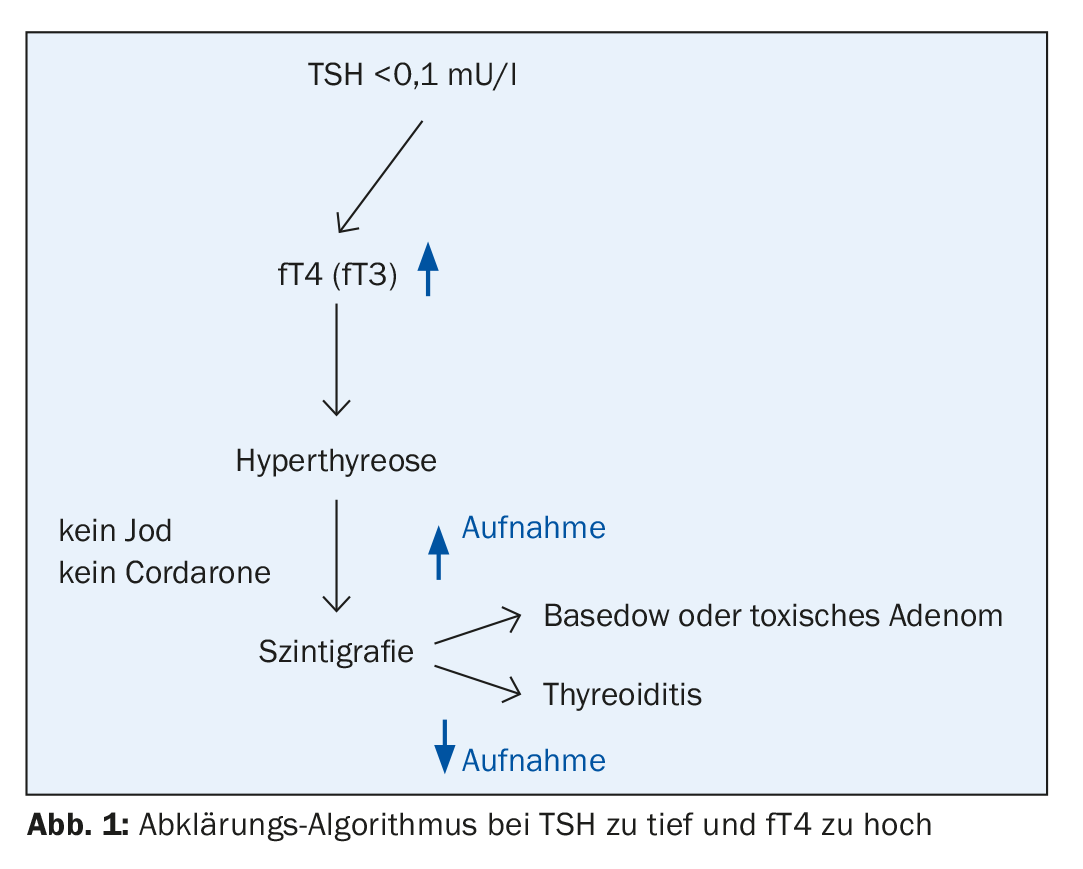
Suppressed TSH values: What should be clarified?
A subclinical form of hyperthyroidism is also usually an incidental finding. According to data from a European study, the frequency varies between 0.5% (in children) and 15% (in persons over 60 years of age), and the differential diagnosis must distinguish between a dysfunction of the thyroid control circuit itself and non-thyroidal causes [4]. Too much or too little thyroid hormone replacement as an iatrogenic cause is relatively common, Prof. Lehmann said. The rate of progression from subclinical to manifest hyperthyroidism varies depending on the pathogenesis and is 61% after two years in the presence of an autonomous nodule (toxic adenoma) [5]. The guidelines recommend scintigraphy in cases of suppressed TSH [6]. This method allows reliable delineation of different causes of subclinical hyperthyroidism.
If TSH values are too low and peripheral thyroid hormones are elevated, this is manifest hyperthyroidism. Clinical features, in addition to impaired performance, typically include weight loss, increased stool frequency, heat intolerance, and sweating, as well as several autonomic symptoms (sleep disturbances, inner restlessness, nervousness). In old age, it often happens that hyperthyroidism is oligosymptomatic, Prof. Lehmann said, and a lack of insight into the disease is also common. Only very rarely does a thyrotoxic crisis develop (hypermetabolism: fever >40 degrees, muscle weakness; sympathetic stimulation: tachycardia, atrial fibrillation, diarrhea), the speaker continues. Etiologically, about 70% of cases are autoimmune thyroiditis (Graves’ disease), for which the Merseburg triad (exopthalmos, goiter, tachycardia) is characteristic [3]. Positive results in antibody measurements are common (TRAK: 70-100%, TPO: 45-80%) and another clinical feature is diffuse goiter and possibly a flow murmur [3]. In about 30% of cases, the etiology is non-autoimmunologic, which is referred to as functional autonomy. In about 2% of cases, other causes of disease are present (drug/contrast agent induced, hyperthyroidism factitia, pituitary thyroid hormone resistance, thyroid malignancy). A characteristic sonographic finding in hyperthyroidism shows diffuse swelling with a hypo-echogenic pattern and mild lobules and rounded thyroid lobes (Fig. 1).
Therapeutic options for Graves’ type hyperthyroidism include thyroidectomy, radioiodine or thyrostatic therapy (carbimazole: Neomercazole®, propylthiouracil), and symptomatic treatment (β-blockers: propanolol or atenolol). Primarily, treatment with thyrostatic agents is indicated, and definitive therapy (radioiodine or surgery) is recommended in cases of treatment resistance or hyperthyroidism recurrence [7]. The thyrostatic carbimazole causes a reduction in thyroid hormone levels by blocking the thyroid peroxidase responsible for the formation of fT3 and fT4. About one-third of patients treated with drugs have a durable remission, one-third have a relapse after a short time, and one-third have a relapse after a longer time interval.
Drug-induced thyroid dysfunction
Amiodarone is a class III antiarrhythmic agent that has good efficacy in patients with cardiac arrhythmias, but may cause thyroid dysfunction as a side effect. Approximately 12% of patients treated with amiodarone (Cordarone®) develop thyroid dysfunction as a side effect, with either hypothyroidism (10%) or hyperthyroidism (2%). The iodine concentration of amiodorone is very high (30%), which can lead to a 200-fold increase in iodine uptake in the organism. The recommended daily iodine requirement for adults is 150 µg, due to amiodarone (50% of the contained iodine is absorbed) the daily dose can be up to 30 mg iodine. Since the half-life is 40 to 100 days due to the lipophilic properties of the active ingredient, the iodine remains in the body for a relatively long time. Amiodarone biochemically affects thyroid hormone levels in multiple ways by blocking fT4-fT3 conversion and uptake of T4 by the cell as well as intranuclear fT3 receptor binding.
As a side effect of taking amiodorone (Cordarone®), the following forms of hypothyroidism or hyperthyroidism may occur [3]: Autoimmune hypothyroidism (AIH), autoimmune hyperthyroidism type 1 (AIT type 1) and type 2 (AIT type 2). AIH is characterized by having a lot of iodine and positive results on thyroid antibody measurements. Type 1 AIT is characterized by excessive T4 synthesis, normal to increased vascularity especially in iodine deficient areas, and usually preexisting thyroid disease. AIT type 2 is inflammatory-destructive, has a decrease in vascularity, usually there is no preexisting thyroid disease, and later hypothyroidism may develop.
In contrast to amiodorone (Cordarone®), the thyroid-related side effect risk of dronedarone (Multaq®), also an arrhythmic agent, is not problematic [3,8]. Regarding therapeutic options, thyroid hormone substitution can be performed in AIH. For AIT type 1, prescription of carbimazole 40-60 mg per day or perchlorate 1 g per day (50 drops) for a period of 30-40 days is indicated. In AIT type 2, prednisone should be prescribed due to the inflammatory-destructive processes, in an initial dose of 30-40 mg and dose reduction within three months [3].
Thyroid incidentalomas: mostly benign
The sensitivity of routine radiologic studies (e.g., sonography) is much higher than that of palpitations. The rate of detected thyroid nodules in a 60-year-old person is 50%, whereas with palpitation it is only about 5% [9]. According to the literature, the prevalence of thyroid incidentalomas is up to 60% [10]. The majority of thyroid incidentalomas are benign (93.5-96%), and malignant thyroid tumors are rare (4-6.5%) [3]. FDG-PET studies have high specificity; incidentaloma is detected in 2.3% cases, of which 47% are malignant tumors [11].
The following features are characteristic of malignant thyroid incidentalomas [12]: hypoechogenic, microcalcifications, central vascularity, irregular boundary, incomplete halo, mixed cystic-solid, documented enlargement of a nodule. The following features are indicative of benign thyroid incidentalomas: hyperechogenic, large and gross calcifications (except medullary thyroid carcinoma), peripheral vascularity, comet tail shadowing. Since a thyroid incidentaloma with a diameter of > 8 mm is found in 40% of all persons over 50 years of age during ultrasound examinations, but the probability that it is a malignant tumor is very low (mortality rate due to thyroid carcinoma is 5:1,000,000), thyroidectomy as a standard procedure is disproportionate and does not make sense, explains Prof. Lehmann.
Source: FOMF Internal Medicine, Update Refresher, December 4-8, 2018, Zurich.
Literature:
- Binz K, Beise U, Huber F: mediX Guideline Thyroid Diseases. Last updated 9/2017, www.medix.ch, last accessed 05/12/2018.
- Dayan CM, Daniels GH: Chronic autoimmune thyroiditis. N Engl J Med 1996; 335: 99-107.
- University Hospital Zurich USZ. Clinic of Endocrinology, Diabetology and Clinical Nutrition. Prof. Roger Lehmann, MD. Slide presentation at FOMF Internal Medicine (unpublished clinical data), Dec 04, 2018.
- Santos Palacios S, Pascual-Corrales E, Galofre JC: Management of Subclinical Hyperthyroidism. Int J Endocrinol Metab 2012; 10(2): 490-496.
- Schouten BJ, et al: Subclinical thyrotoxicosis in an outpatient population – predictors of outcome. Clinical Endocrinology 2011; 74: 257-261.
- Surks MI, et al: Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA 2004; 291(2): 228-238.
- Krull I, Brändle M: Hyperthyroidism: Diagnosis and Therapy. Switzerland Med Forum 2013; 13(47): 954-960.
- Arzneimittel-Kompendium der Schweiz: https://compendium.ch, last accessed 05.12.2018.
- Mazzaferri EL: Management of a solitary thyroid nodule. N Engl J Med 1993; 328 (8): 553-559.
- Iyer NG, et al: Thyroid incidentalomas: to treat or not to treat. Eur Arch Oto-Rhin-Laryngology 2010; 267: 1019-1026.
- Cohen MS, et al: Risk of malignancy in thyroid incidentalomas identified by fluorodeoxyglucose-positron emission tomography. Surgery 2001; 130(6): 941-946.
- American Thyroid Association: Management Guidelines for Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2009; 19: 1167-1214.
HAUSARZT PRAXIS 2019; 14(1): 35-37