Perioperative myocardial infarction/injury (PMI) after noncardiac surgery is a common cardiac complication. A better understanding of the underlying etiology and consequences is urgently needed.
Perioperative myocardial infarction or perioperative myocardial injury (PMI) is increasingly recognized as a common perioperative cardiac complication after major noncardiac surgery and contributes significantly to postoperative mortality [2–6]. Because of the strong analgesia administered in the perioperative period, most PMI occur without typical ischemic symptoms and are therefore overlooked in routine clinical practice without active monitoring [2–4,7,8]. Because mortality associated with asymptomatic PMI is comparable to that associated with symptomatic PMI, active surveillance of PMI with preoperative and postoperative measurements of cardiac troponin (cTn) is increasingly advocated as part of routine clinical care to enable early detection and treatment [9–12]. Recently, the European Society of Cardiology issued a class IB recommendation for such active monitoring of PMI [12].
Previous studies in which active surveillance of PMI has been performed have shown that PMI is not a homogeneous disease but a heterogeneous syndrome with several different underlying etiologies, including type 1 myocardial infarction (T1MI) which is caused by plaque rupture, type 2 myocardial infarction (T2MI), which is caused by a supply-demand mismatch, tachyarrhythmia, acute heart failure, and primary extracardiac PMI, which may be due to, eg. e.g., due to severe sepsis or pulmonary embolism (PE). [2–4,13–16]. A better understanding of the underlying etiology is a prerequisite for targeted preventive and/or therapeutic interventions for PMI in individual patients [9,17,18]. In centrally determining the etiology of PMI, unexpected differences in incidence and short-term mortality at 30 days were recently observed in a pilot study, further emphasizing the clinical importance of detailed phenotyping [16]. However, little is known about the long-term consequences of the various PMI etiologies [3]. Therefore, a recent prospective multicenter study investigated the serious adverse cardiac events (MACE) and all-cause mortality associated with the different centrally assessed PMI etiologies within one year [1].
Population
Between May 2014 and June 2018, a total of 10 772 patients were enrolled, of whom 7754 were eligible for this analysis. Consecutive patients undergoing major inpatient noncardiac surgery at three hospitals (University Hospital Basel, Kantonsspital Aarau, both in Switzerland, and Instituto do Coracao, InCor, Universidade de Sao Paulo, Brazil) eligible for the institutional active PMI surveillance and response program for high-risk patients undergoing major inpatient noncardiac surgery were prospectively enrolled [2].
Patients were screened if they had an increased risk of mortality, were ≥65 years of age or ≥45 years of age, and had coronary artery disease, peripheral arterial disease, or stroke and underwent inpatient noncardiac surgery with a planned postoperative stay of ≥24 hours. Plasma concentrations of cTn were measured within 30 days before surgery and on postoperative days one and two as part of active monitoring and when clinically indicated after surgery. A twelve-lead electrocardiogram (ECG) was performed on the day PMI was detected and when clinically indicated.
Assessment of PMI etiology
The etiology of PMI, as determined by an active surveillance and response program, was centrally assessed by two independent physicians based on all information obtained during clinically indicated PMI screening, including cardiac imaging, in consecutive high-risk patients undergoing major noncardiac surgery in a prospective multicenter study. The etiology of PMI was hierarchically divided into “extracardiac,” when caused by a primarily extracardiac condition such as severe sepsis or pulmonary embolism, and “cardiac,” further subdivided into type 1 myocardial infarction (T1MI), tachyarrhythmia, acute heart failure (AHF), or probable type 2 myocardial infarction (lT2MI).
Endpoints
The primary end points were the occurrence of MACE and deaths of all types according to different PMI etiologies within one year. Deaths were classified as cardiovascular or noncardiovascular according to the most recent guidelines [19]. MACE was defined as a composite end point of acute myocardial infarction, AHF, life-threatening arrhythmias, and cardiovascular death. Follow-up began after surgery on the day of the procedure. To avoid definitional bias, index PMI was not counted as an end point event in patients with PMI classified as T1MI or AHF; instead, follow-up for acute myocardial infarction or AHF began after postoperative day 3. A composite of MACE and all-cause death was a secondary end point.
To optimize the event-to-noise ratio for the time-to-event and prognostic analyses, the occurrence of MACE and all-cause death at 120 days was chosen as the secondary end point and used for the prognostic and time-to-event analyses because a previous study suggested a vulnerable period of 120 days after noncardiac interventions [20].
Characteristics of PMI etiologies
PMI occurred in 1016/7754 patients (13.1%), of whom 109/1016 (10.7%) were centrally classified as primarily extracardiac. PMI were classified as T1MI in 71/1016 patients (7.0%), tachyarrhythmia in 47/1016 patients (4.6%), and AHF in 39/1016 (3.8%). Of the patients diagnosed with T1MI, coronary angiography was performed within seven days in 42/71 patients, within 30 days in 47/71 patients, and a decision was made without coronary angiography in 24 patients. The remaining 750/1016 (73.8%) were classified as lT2MI.
Baseline characteristics differed among the prespecified PMI etiologies, with, for example, known CHD being more common in T1MI and AHF than in all other PMI etiologies. Additional criteria for a diagnosis of spontaneous AMI were present in 260/1016 (25%) of patients with PMI, eg, ischemic symptoms or dyspnea in 143/1016 (14%), again with large differences between PMI etiologies. In 34/1016 patients (3%), PMI without a preoperative cTn value was assessed by the change between postoperative values.
Follow-up
Follow-up was completed in 7754/7833 patients (99%) with a median duration of 388 days. At least one MACE occurred in 684/7754 patients after one year (8.8%). A total of 818/7754 patients died within 1 year (10.5%), with death occurring during the index hospitalization in 154/817 patients. MACE or death as an overall cause occurred in 1160/7754 patients (15.0%).
MACE according to PMI depending on etiology
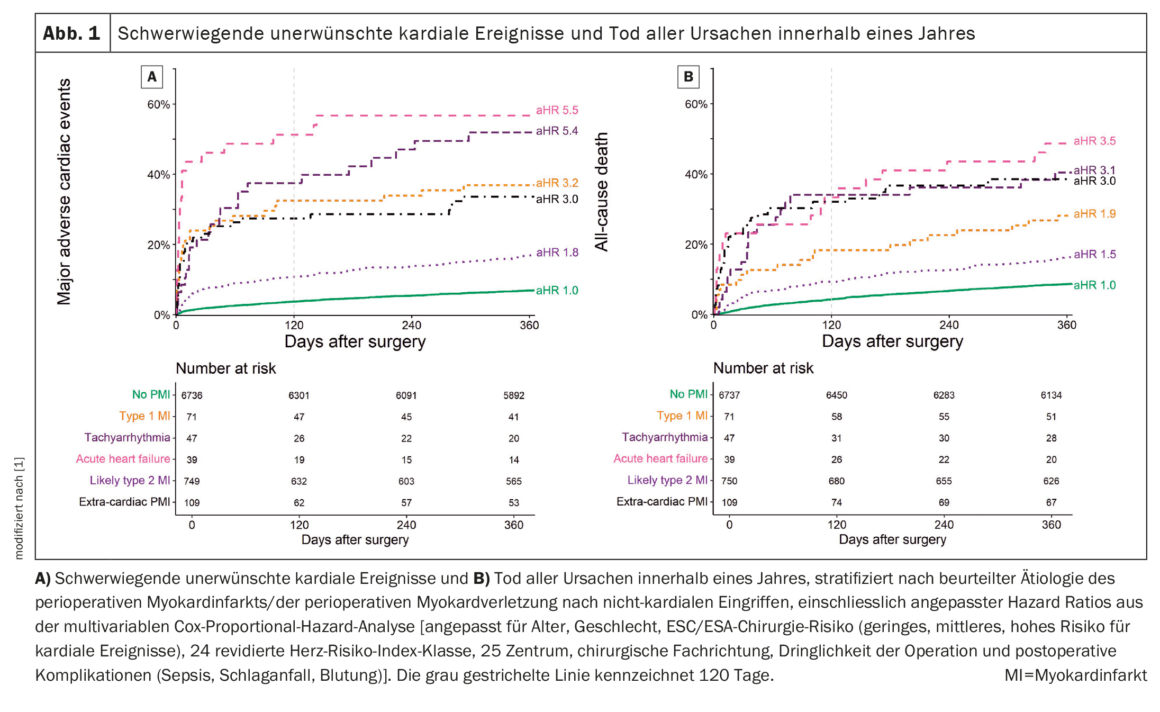
For all PMI etiologies, the 1-year MACE rate was significantly increased compared with patients without PMI. In patients without PMI (7%, 95% CI 6%-8%), and within PMI etiology, there were significant differences (Fig. 1A) [1]: Extracardiac PMI, T1MI, tachyarrhythmia, and AHF were associated with very high rates of MACE [30% (95% CI 20-47), 37% (95% CI 24-47), 49% (95% CI 34-75), and 56% (95% CI 38-70), respectively], and lT2MI was also associated with high rates of MACE (17%, 95% CI 22-28). The associations were confirmed in multivariable analysis with adjusted hazard ratios (aHRs) of 3.0 (2.0-4.5), 3.2 (95% CI 2.1-4.8), 5.4 (3.5-8.4), and 5.5 (3.5-8.7) for extracardiac PMI, T1MI, tachyarrhythmia, and AHF, respectively, and 1.8 (1.5-2.2) for lT2MI.
A similar association was seen for MACE at 120 days, with numerically higher aHR in multivariable analysis compared with 1-year associations but lower power because of fewer events. Time from surgery to onset of MACE within 120 days differed significantly between PMI etiologies, from a median of three days after AHF to 13 days after lT2MI and 26 days in patients without PMI. When the total number of MACE stratified by the etiology of PMI was examined, multiple events occurred in 3% to 23% of patients, compared with 1% in patients without PMI. The association of PMI etiology with MACE was stronger within the first 120 days than after one year.
Total mortality according to PMI
All PMI etiologies had an increased 1-year all-cause mortality rate compared with patients without PMI. There were significant differences in patients without PMI (9%, 95% CI 8-9%), and within PMI etiologies (Fig. 1B) [1]: extracardiac PMI, T1MI, tachyarrhythmia, and AHF were associated with high mortality rates [38% (95% CI 29-47), 28% (95% CI 17-38), 40% (95% CI 25-53), and 49% (95% CI 30-62), respectively], and lT2MI was also associated with high mortality rates (17%, 95% CI 14-20). This relationship was confirmed in the multivariable analysis.
Prediction model for lT2MI
MACE or all-cause death occurred in 117/750 patients with lT2MI within 120 days. The presence of chest pain or dyspnea, an absolute cTn increase of >2 × 99th percentile compared with baseline, high-risk surgery according to the ESC/ESA surgical risk score, and nonelective surgery were associated with an increased risk of MACE, whereas bleeding was associated with a lower risk of MACE. Internal validation showed good agreement between predicted and observed event rates after bootstrapping with 1000 iterations and a Brier score of 0.16.
The prognostic model showed a moderate AUC of 0.71 (95% CI 0.66-0.76). Compared to the maximum RCRI (available in 747/750 cases), ASA class (available in 718/750 cases), and absolute hs-cTnT-delta (available in 564/750 cases), the prognostic model showed the numerically highest AUC, which was statistically different from ASA class and hs-cTnT-delta (p=0.003) and comparable to RCRI (p=0.195). Combining the prognostic model with ASA class or RCRI further significantly increased AUC compared to RCRI or ASA class alone (model+ASA 0.75, 95% CI 0.71-0.80, p<0.001; model+RCRI 0.75, 95% CI 0.70-0.80, p<0.001). After categorization by the prognostic model, the lT2MI-PMI “very high risk” group had a rate of MACE or death of 31% (comparable to PMI of T1MI), whereas the “low risk” group had rates of 7% (comparable to patients without PMI).
After one year, high rates of MACE and all-cause deaths.
The large prospective international multicenter study centrally assessed the etiology of PMI detected during active surveillance in high-risk patients undergoing major noncardiac surgery and closely monitored MACE during long-term follow-up. At one year, most PMI etiologies have unacceptably high rates of MACE and all-cause death, underscoring the urgent need for more intensive treatments.
Literature:
- Puelacher C, et al: Long-term outcomes of perioperative myocardial infarction/injury after non-cardiac surgery. EurHeartJ 2023.
https://doi.org/10.1093/eurheartj/ehac798 - Puelacher C, Lurati Buse G, Seeberger D, et al: Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation 2018;137: 1221-1232. https://doi.org/10.1161/CIRCULATIONAHA.117.030114
- Botto F, Alonso-Coello P, Chan MT V, et al: Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014;120: 564-578.
https://doi.org/10.1097/ALN.0000000000000113 - Devereaux PJ, Biccard BM, Sigamani A, et al: Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017;317: 1642-1651.
https://doi.org/10.1001/jama.2017.4360 - Landesberg G, Mosseri M, Zahger D, et al: Myocardial infarction after vascular surgery: the role of prolonged stress-induced, ST depression-type ischemia. J Am Coll Cardiol 2001;37:1839-1845. https://doi.org/10.1016/S0735-1097(01)01265-7.
- Landesberg G, Shatz V, Akopnik I, et al: Association of cardiac troponin, CK-MB, and postoperative myocardial ischemia with long-term survival after major vascular surgery. J Am Coll Cardiol 2003;42: 1547-1554. https://doi.org/10.1016/j.jacc.2003.05.001
- Devereaux PJ, Xavier D, Pogue J, et al.: Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med 2011;154: 523–528. https://doi.org/10.7326/0003-4819-154-8-201104190-00003
- Devereaux PJ, Chan MT V, Alonso-Coello P, et al.: Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012;307: 2295–2304. https://doi.org/10.1001/jama.2012.5502
- Thygesen K, Alpert JS, Jaffe AS, et al.: Fourth universal definition of myocardial infarction (2018). Eur Heart J 2019;40: 237–269. https://doi.org/10.1093/eurheartj/ehy462.
- Duceppe E, Parlow J, MacDonald P, et al.: Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol 2017;33: 17–32. https://doi.org/10.1016/j.cjca.2016.09.008
- Gualandro DM, Yu PC, Caramelli B, et al.: 3rd guideline for perioperative cardiovascular evaluation of the Brazilian society of cardiology. Arq Bras Cardiol 2017;109: 1–104. https://doi.org/10.5935/abc.20170140
- Halvorsen S, Mehilli J, Cassese S, et al.: 2022 ESC guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery. Eur Heart J 2022;43: 3826–3924. https://doi.org/10.1093/eurheartj/ehac270.
- Gualandro DM, Campos CA, Calderaro D, et al.: Coronary plaque rupture in patients with myocardial infarction after noncardiac surgery: frequent and dangerous. Atherosclerosis 2012;222: 191–195. https://doi.org/10.1016/j.atherosclerosis.2012.02.021
- Duvall WL, Sealove B, Pungoti C, et al.: Angiographic investigation of the pathophysiology of perioperative myocardial infarction. Catheter Cardiovasc Interv 2012;80: 768–776. https://doi.org/10.1002/ccd.23446
- Sheth T, Natarajan MK, Hsieh V, et al.: Incidence of thrombosis in perioperative and non-operative myocardial infarction. Br J Anaesth 2018;120: 725–733. https://doi.org/10.1016/j.bja.2017.11.063
- Puelacher C, Gualandro DM, Lurati Buse G, et al.: Etiology of peri-operative myocardial infarction/injury after noncardiac surgery and associated outcome. J Am Coll Cardiol 2020;76: 1910–1912. https://doi.org/10.1016/j.jacc.2020.08.043
- Landesberg G, Beattie WS, Mosseri M, et al.: Perioperative myocardial infarction. Circulation 2009;119: 2936–2944. https://doi.org/10.1161/CIRCULATIONAHA.108.828228.
- Howell SJ, Brown OI, Beattie WS: Aetiology of perioperative myocardial injury: a scientific conundrum with profound clinical implications. Br J Anaesth 2020;125: 642–646. https://doi.org/10.1016/j.bja.2020.08.007.
- Hicks KA, Tcheng JE, Bozkurt B, et al.: 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials. Circulation 2015;132: 302–361.
- Sazgary L, Puelacher C, Lurati Buse G, et al: Incidence of major adverse cardiac events following non-cardiac surgery. Eur Heart J Acute Cardiovasc Care 2020;10: 550-558. https://doi.org/10.1093/ehjacc/zuaa008.
CARDIOVASC 2023; 22(1): 46-48