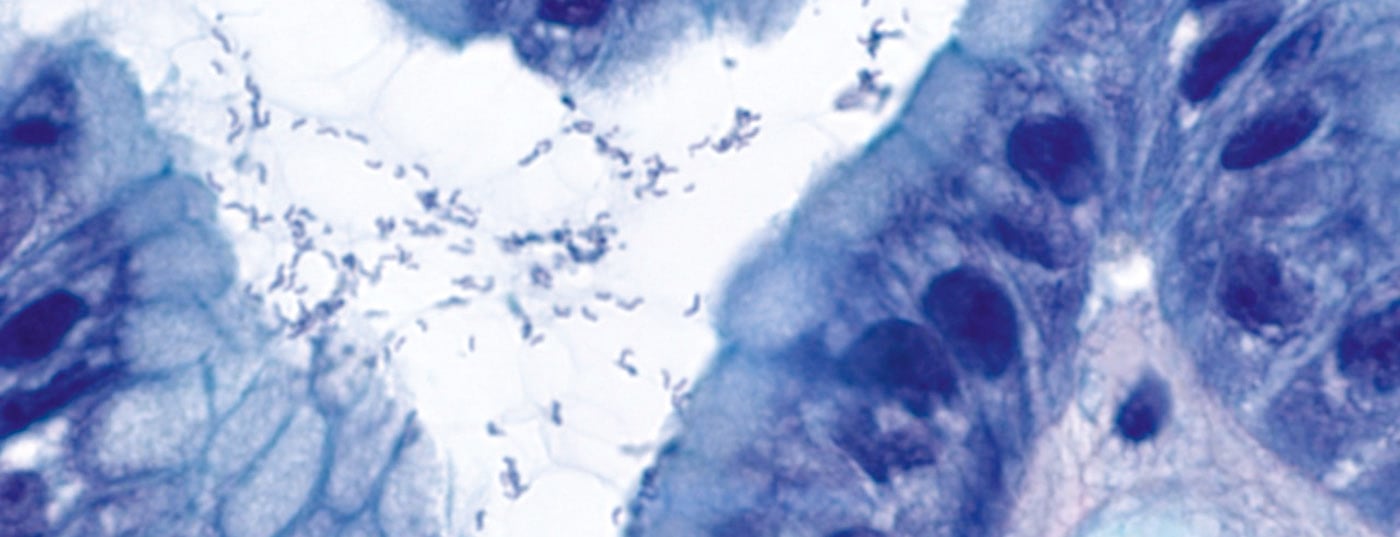
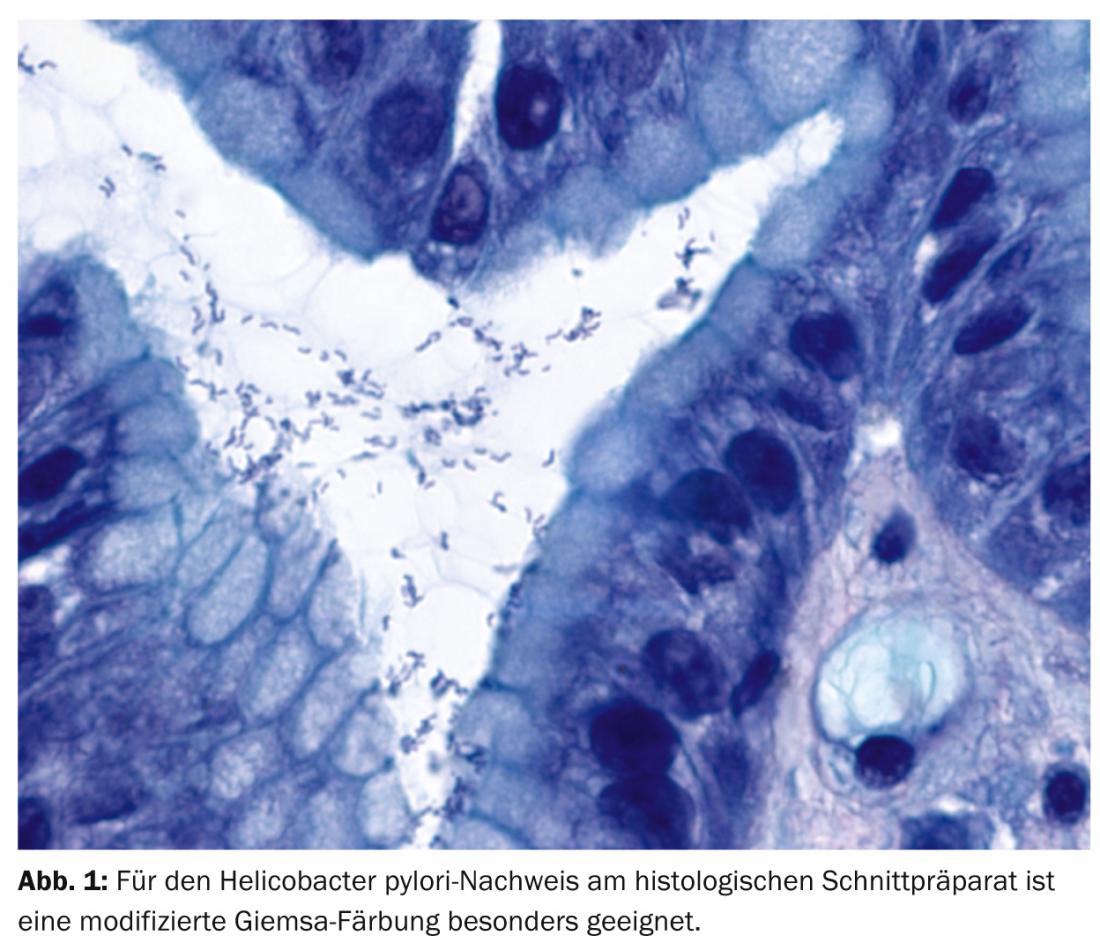
MALT lymphomas most commonly affect the stomach. Causally, there is a strong association with Helicobacter pylori (Hp) gastritis (over 90% of patients). With adequate antibiosis, Hp can be eradicated, leading to complete remission in nearly 80% of patients with early-stage MALT lymphoma. The diagnosis of MALT lymphoma falls largely within the domain of morphologic evaluation by the pathologist. The specific t(11;18)(q21;q21) translocation has conceptual, pathophysiological, prognostic, predictive and increasingly therapeutic significance.
The mucosa-associated lymphoid tissue (MALT) is a second, biologically different compartment in addition to the nodal somatic lymphoid tissue. The conceptual importance and better understanding of lymphomas of extranodal origin versus nodal equivalents originate from the work of Isaacson and Wright 30 years ago with the initial description of MALT lymphoma (MALTL) in the stomach, lung, salivary gland, and thyroid [1]. The recognition of variable frequencies of genetic aberrations in MALTL of different provenances gives increasing importance to organ specificity. Among many others, however, the stomach remains the organ most commonly affected by MALTL.
The concept of infection
Strictly speaking, the development of the MALT concept actually begins another 20 years earlier with the description of “Mediterranean lymphoma” in the small intestine, characterized by malabsorption and formation of abortive immunoglobulin (Ig) consisting of defective α-heavy chains without light chains, for which the term “α-chain disease” was coined [2].
In lymphoma, later also known as “immunoproliferative small intestinal disease” (IPSID), early stages could be brought into remission under antibiosis, thus giving birth to the idea of antigen (Ag)-triggered, antibiotic-sensitive tumors [3]. With documented association with Helicobacter pylori (Hp) gastritis in over 90% of MALTL patients, the causal link between antecedent Hp infection and subsequent tumor development was rapidly
established [4].
Helicobacter pylori
Also, 30 years ago, Hp (Fig. 1) was first described as “unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration” by Barry Marshall and John Robin Warren [5]. The two were awarded the Nobel Prize in Medicine in 2005 for this discovery. Specifically, Hp infection causes chronic gastritis and leads to acquisition of mucosa-associated lymphoid tissue, from which clonal selection can result in MALTL.
Hp-triggered indirect and direct Ag stimulation of B lymphocytes is mediated by Ag-presenting cells and Hp-specific intratumoral T lymphocytes. The immune response, i.e., production of anti-Hp antibodies and idiotypic Ig, cannot efficiently eliminate Ag. However, on the contrary, with adequate antibiosis, Hp can be eradicated and complete remission of lymphoma can be achieved in nearly 80% of early-stage MALTL patients [6].
A better understanding of the revolutionary therapeutic paradigm shift to successfully treat a tumor with antibiotics arises from the biological continuum of lymphoproliferative infiltration in the stomach that begins in Hp gastritis and ends in highly malignant transformed B-cell lymphoma.
Hp positive/Hp negative
In the absence of a gold standard, combined testing for Hp is performed in symptomatic patients, and detection is often false-negative under continuous H2-blocker therapy. Clinically, different Hp types differ in terms of virulence, with Cag-A+ strains associated with significantly higher patient morbidity.
Approximately 10% of MALTL patients are Hp-negative; they are likely to have a worse prognosis in terms of overall survival. Understandably, eradication therapy alone is rarely successful in curing them, so that antitumor therapy is necessary. Helicobacter heilmannii plays a role in only about one per thousand of all Hp infections in humans.
Hp vaccination
With an average incidence of Hp infection of 30% and the associated risk of developing B gastritis, gastric ulcer, MALTL, or even gastric carcinoma, the urgency of prophylaxis is understood. To date, however, Hp vaccines have only been used in mice in the laboratory. Nevertheless, a significant reduction in bacterial colonization can be achieved in the experiment, which also proves that vaccines trigger an immune response in the host and that it therefore has eradication potential. However, the natural immune response remains bound to regulatory T cells, which in turn limits Hp elimination. More may be expected from future vaccination concepts that could bypass or override host-bound immune regulation [7].
Morphology
In the routine approach of the pathologist, the diagnosis of MALTL falls largely within the domain of morphology. Thus, gastric biopsy shows diffuse lymphoproliferate in mucosa and submucosa with quite circumscribed foci of infiltration in the surface epithelium and glandular body, the so-called lymphoepithelial lesions (Fig. 2).
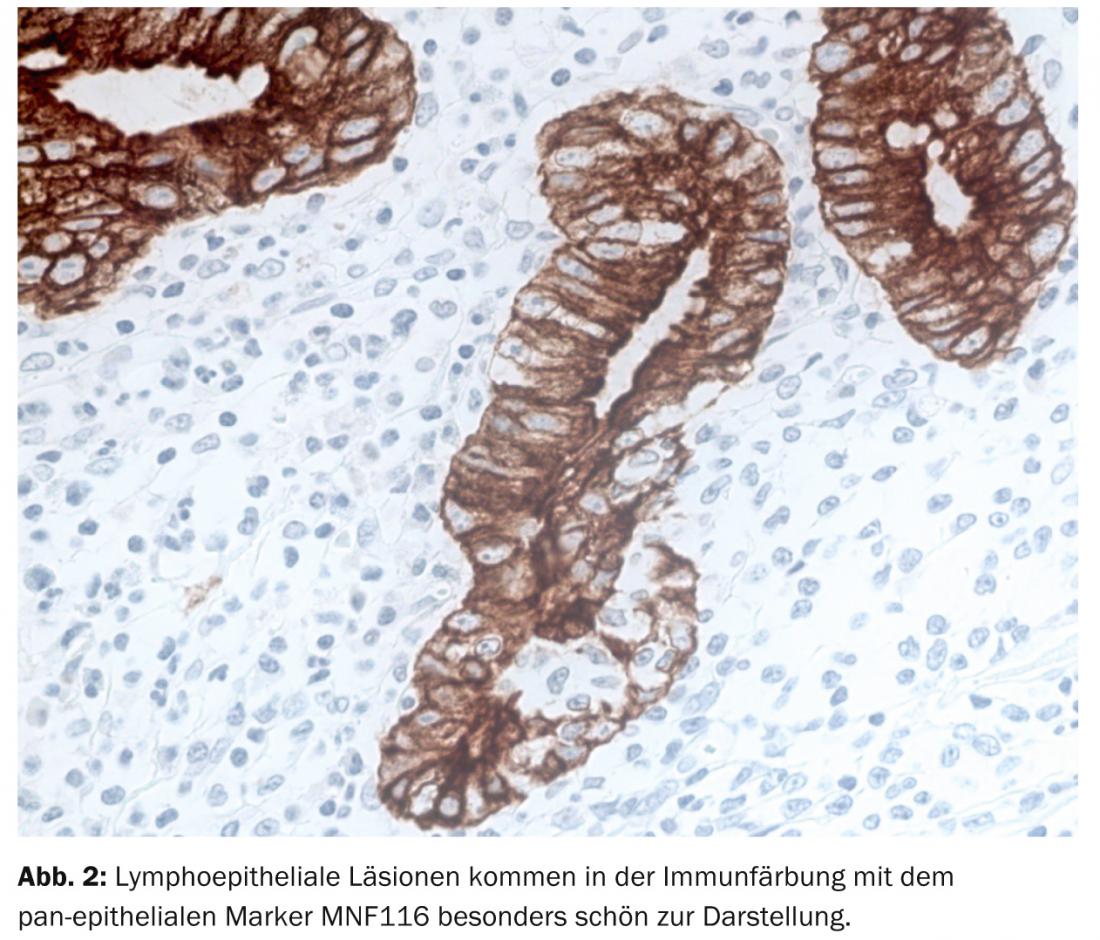
Intratumorally, residual reactive lymphoid follicles are found, which are first excluded from the tumor, but later colonized by it. Cytomorphologically, tumor cells are small to medium-sized and variable in centrocytoid, lymphocytoid, or monocytoid shape. In addition, MALTL shows plasma cell differentiation typically directed against the surface epithelium. As with other low-malignant lymphomas, transformation to blastic lymphoma is possible in MALTL.
Immune profile
According to the valid WHO classification 2008, MALTL has no specific immune profile and thus cannot be defined immunophenotypically in an affirmative manner. Rather, it simply remains as a last option in differential diagnosis against other low-malignant B-cell lymphomas, quasi per exclusionem. Positivity is obligatory for CD20 and, as an expression of plasma cell differentiation, usually even somewhat more pronounced for CD79a, whereas the detectability of monotypic Ig depends strongly on biopsy quality. Typically, positivity is found for CD21, CD35, CD43, Bcl-10, IRTA-1, and T-bet, but all of these may be expressed as nonspecific markers in other lymphoma subtypes. Ultimately, probably most helpful for diagnosis is the lack of detection of CD10 or of cyclin D-1 and SOX-11 in differentiation against follicular lymphoma and mantle cell lymphoma.
Molecular markers
In the interpretation of equivocal lymphoproliferative B-cell infiltrations in the stomach, although clonality analysis in PCR has been shown to have differential diagnostic importance, but clonality detection is ultimately irrelevant to the immediate therapeutic consequences pragmatically, as antibiotic Hp eradication is performed for both polyclonal Hp gastritis and monoclonal MALTL in the Ag-dependent early stage. As an interjection, it is worth noting that “clonal gastritis” is, after all, diagnosed much more frequently by gastroenteropathologists than it would be properly recognized by hematopathologists [8].
Similarly, the detection of MALT-1 gene disruption plays no role in the routine diagnosis of MALTL, although I will discuss the more specific significance of the t(11;18)(q21;q21) translocation in gastric MALTL.
MALTL-specific molecular biology
Diagnostically, the recurrent reciprocal t(11;18)(q21;q21) translocation leading to the chimeric fusion hybrid BIRC3(API2)-MALT1 is the most specific structural aberration in gastric MALTL [9], which can be detected in 30-50% of patients by FISH analysis (Fig. 3).
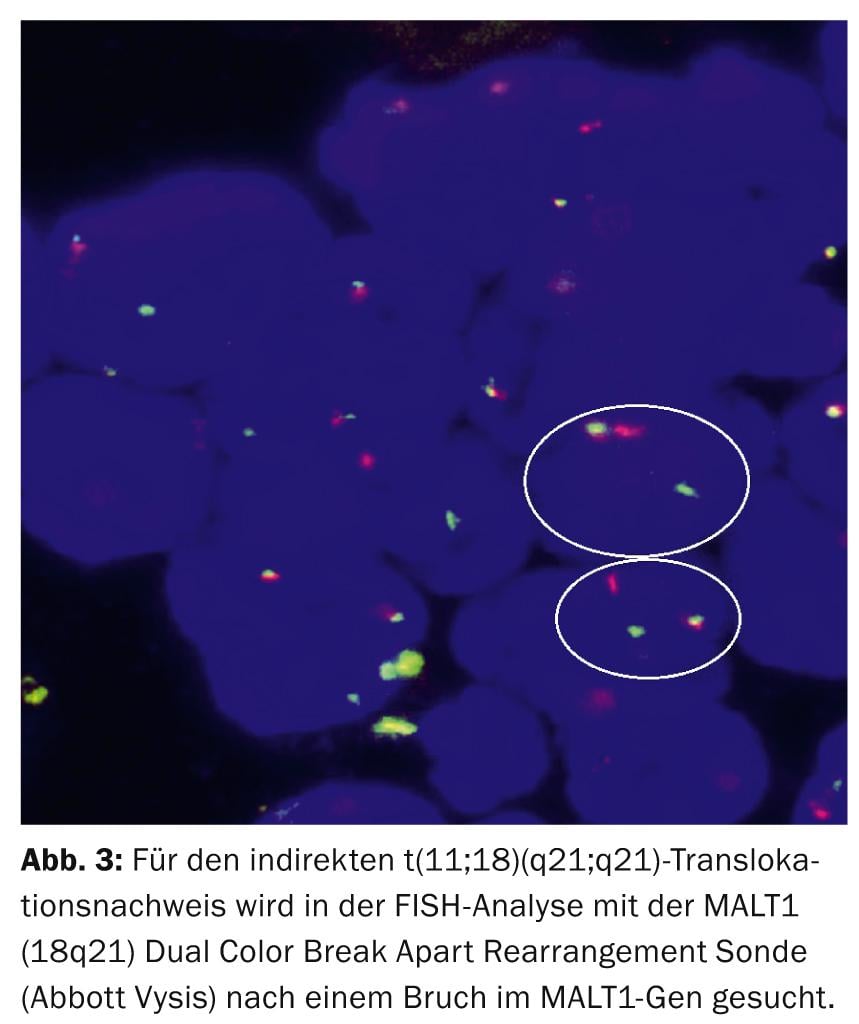
Pathophysiologically, t(11;18)/BIRC3(API2)-MALT1 synergistically with the t(1;14)/BCL10-IGH and t(14;18)/IGH-MALT1 translocations and the TNFAIP3 mutation leads to synchronous apoptosis inhibition and NF-κB activation via the oncogenic interplay of MALT1 and BCL10.
Prognostically, t(11;18) typically characterizes cases with high karyotypic stability as a sole genetic aberration and is protective against potential blastic transformation. The t(11;18) translocation is more frequently detected in MALTL with initial advanced stage and more aggressive progression, tumor progression, and dissemination to other organs and is more frequently associated with Cag-A+ Hp strains.
Predictively, in t(11;18)-positive MALTL, tumorigenesis is not Hp-triggered and consequently 78% of these patients are also refractory to therapy with Hp eradication alone. However, the most common presumed treatment failure is due to the control interval being too short and thus to the impatience of the patient or the oncologist. It is also important to clearly differentiate between persistent Hp colonization of the gastric mucosa, histological evidence of residual lymphoid infiltrates in the repeated gastric biopsy, or merely a persistent B-cell clone in the PCR.
Therapeutically, MALT1 inhibitors have recently been tested directly for antitumor activity in vitro [10].
MALTL non-specific molecular biology.
In contrast, the translocations t(3;14)/FOX-P1-IGH, t(3;14)/BCL6-IGH, and t(8;14)/C-MYC-IGH are found exclusively in blastically transformed MALTL as nonspecific structural genetic aberrations. Frequent numerical anomalies, such as trisomy 3 associated in 80% with trisomies 11, 12 or 18, microsatellite instability/allelic imbalance, TP53 inactivity or p16 deletion remain to be mentioned for the sake of completeness.
Take home messages
- Highly malignant B-cell lymphoma of the stomach may be considered a blastic transformation if isoclonal Ig rearrangement is detected and the gene expression signature is identical to that in MALTL, although the molecular mechanisms for this have not been fully elucidated.
- Helicobacter heilmannii (Hh, Gastrospirillium hominis) plays a role in only about one per thousand of all Helicobacter infections in humans. Rare cases with Hh-associated MALT lymphoma can be brought into complete remission with antibiotic eradication therapy, analogous to Hp.
- “Tumor is a genetic diesease”: the t(11;18)(q21;q21)/BIRC3(API2)-MALT1 translocation is the major molecular biomarker of gastric MALT lymphoma and has diagnostic, conceptual, pathophysiological, prognostic, predictive and therapeutic significance.
PD Dr. med. Sergio B. Cogliatti
Literature:
- Isaacson PG, Wright DH: Extranodal malignant lymphoma arising from mucosa-associated lymphoid tissue. Cancer 1984; 53: 2515-2524.
- Frand U, et al: Malignant lymphoma in Israel – an epidemiological study on 399 cases. Harefua 1963; 65: 83-86.
- Galian A, et al: Pathological study of alpha-chain disease with special emphasis on evolution. Cancer 1977; 39: 2081-2101.
- Doglioni C, et al: High incidence of primary gastric lymphoma in northeastern Italy. Lancet 1992; 339: 834-835.
- Marshall BJ, Warren JR: Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984; 323: 1311-1315.
- Wotherspoon AC, et al: Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobater pylori. Lancet 1993; 342: 575-577.
- Czinn SJ, Blanchard T: Vaccinating against Helicobater pylori infection. Nat Rev Gastroenterol Hepatol 2011; 8: 133-140.
- Hummel M, et al: Wotherspoon criteria combined with B cell clonality analysis by advanced polymerase chain reaction technology discriminates covert gastric marginal zone lymphoma from chronic gastritis. Gut 2006; 55: 782-787.
- Mathijs B, Marynen P: t(11 ;18)(q21 ;q21) BIRC3/MALT1. Atlas Genet Cytogenet Oncol Haematol 2002; 6: 34-36.
- Burgess DJ: Assault on MALT1. Nature Reviews Cancer 2013; 13: 80-81.
InFo ONCOLOGY & HEMATOLOGY 2015; 3(1): 22-26.