Despite the increasing number of endovascularly treated cerebral aneurysms, many cases require surgical expertise because of their anatomy, shape, and configuration. Over the past 20 years, physicians and engineers have worked tirelessly to improve surgical and endovascular therapy. A review of modern techniques of surgical and endovascular aneurysm treatment.
Despite the increasing number of endovascularly treated cerebral aneurysms, many cases require surgical expertise because of their anatomy, shape, and configuration. Since the introduction of the surgical microscope and fenestrated clips in the 1960s [1], great efforts have been made to improve surgical access, clip design, and operative procedures, such as in bypass surgery. In addition, new techniques in intraoperative imaging have improved the safety of surgical procedures. Since the 1990s, in the field of endovascular treatment, selectively detachable platinum coils have revolutionized the treatment of cerebral aneurysms [2]. Shortly thereafter, the introduction of remodeling balloons enabled an increase in the packing density of intra-aneurysmal coils and temporary endovascular closure of a ruptured vessel [3]. A decade later, stents were introduced into endovascular therapy, making it possible to treat broad-based aneurysms for the first time [4]. Such aneurysms could previously only be treated surgically. Over the past 20 years, physicians and engineers have worked tirelessly to improve surgical and endovascular therapy. In this article, we describe modern techniques of surgical and endovascular aneurysm treatment.
Surgical techniques
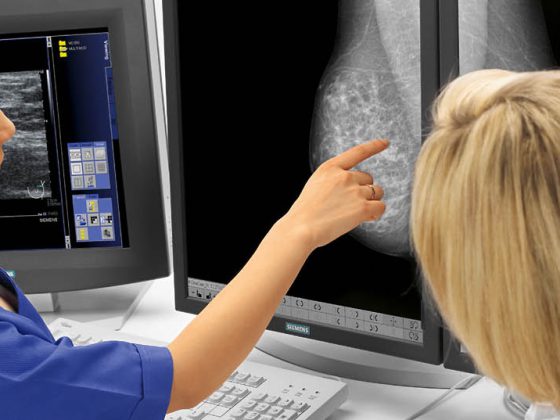
Minimizing the invasiveness of surgical therapy is the focus of modern neurosurgery. In selected patients, keyhole surgery with minimal opening of the cranial vault is possible despite the limited surgical operating space and allows faster postoperative recovery and also better cosmetic results [5]. Minimizing such interventions is made possible in part by modern navigation systems. The technique of clipping aneurysms as well as the design of the clips themselves have also been refined. New titanium aneurysm clips ensure a high closing force and cause hardly any MRI artifacts, which significantly improves the possibilities of non-invasive radiological follow-up [6]. Further developments in the area of non-ferromagnetic clips and imaging algorithms could completely eliminate the problem of artifacts in the future. Vascular reconstructive surgery for complex aneurysms, utilizing multiple and different types of aneurysm clips, is part of modern vascular centers [7,8]. Furthermore, improvements in bypass surgery have expanded surgical treatment options for large and giant aneurysms (>25 mm) [9]. Intraoperative imaging is another area in which recent advances have been made, particularly for assessing the patency of inflow and outflow vessels or perforators during clipping or for assessing complete aneurysm occlusion. Indocyanine green (ICG) and fluorescin (Fl) are intravenous tracers that allow the surgeon to visualize vessels and vascular pathologies during surgery [10–12]. After injection of the tracers, the vessels are visualized by polarized light with microscope-integrated videoangiography (Fig. 1). Another component of modern neurosurgical interventions is neuromonitoring. Here, the function of the brain is monitored electrophysiologically. Cerebral hypoperfusion during neurovascular surgery can be detected and corrected [13]. All these methods and developments promote patient safety and together contribute to definitive and long-term aneurysm treatment.
Endovascular techniques
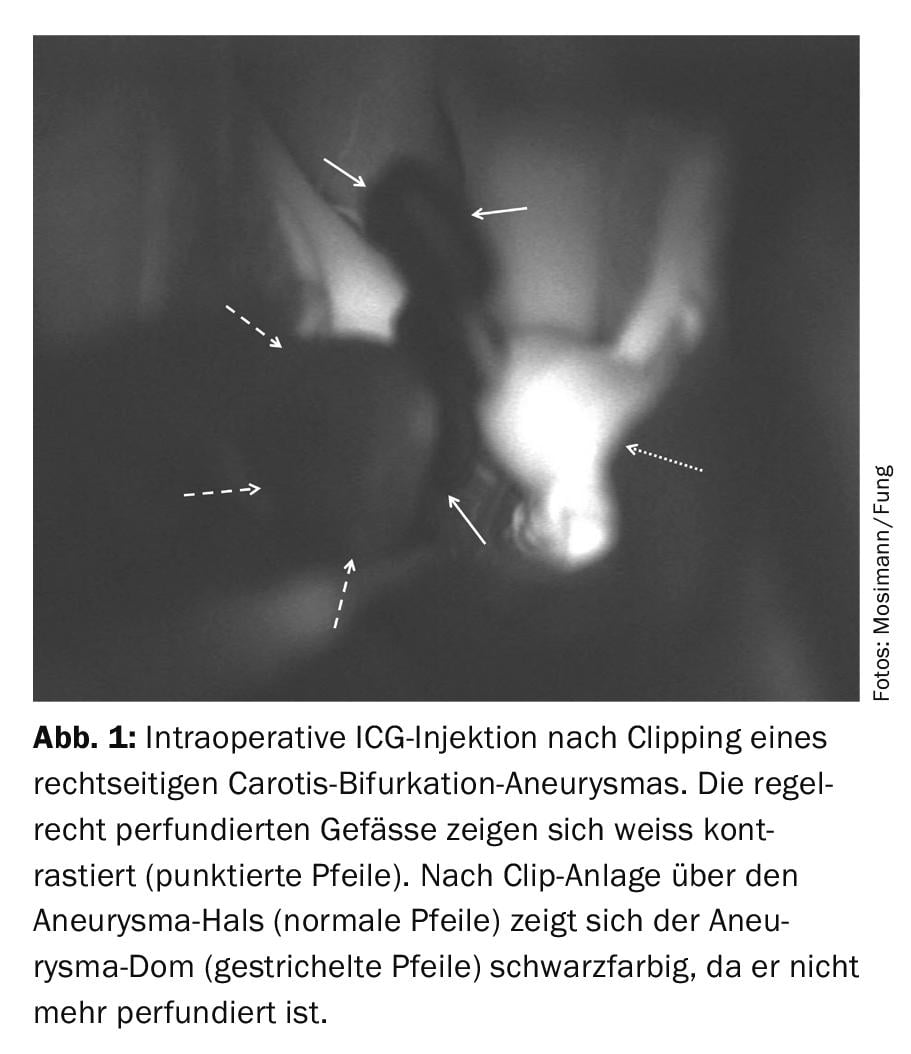
Intrasaccular implants: Standard platinum coils come in various shapes and sizes and are currently the gold standard for the treatment of cerebral aneurysms. The incorporation of hydrogel into the metallic structure of the coils allows higher packing density of the coils by creating a swelling after implantation into the aneurysm. The long-term efficacy of hydrocoils over standard platinum coils in terms of recurrence and re-treatment rates is controversial [14]. Wing-like coils, Medina coils, have recently been introduced with the aim of preventing coil compaction and aneurysm recurrence [15], using additional material at the aneurysm neck to prevent blood inflow into the aneurysm. Here, too, the superiority over standard coils has yet to be demonstrated. The WEB device is the latest intrasaccular device tested in large clinical registries [16]. It consists of a nitinol cage of self-expanding narrow meshes that promotes intrasaccular thrombosis, leading to flow interruption into the aneurysm (Fig. 2) . Depending on the anatomy, this device should be used to treat large and broad-based aneurysms of the bifurcations and terminal arteries (media or carotid bifurcation, basilar tip aneurysm). The majority of all broad-based aneurysms require one or more stents to hold the coils in the aneurysm sac and to prevent recanalization. Because WEB implantation does not leave significant metallic structures in the aneurysm-bearing vessel, long-term dual platelet aggregation inhibition is not required.
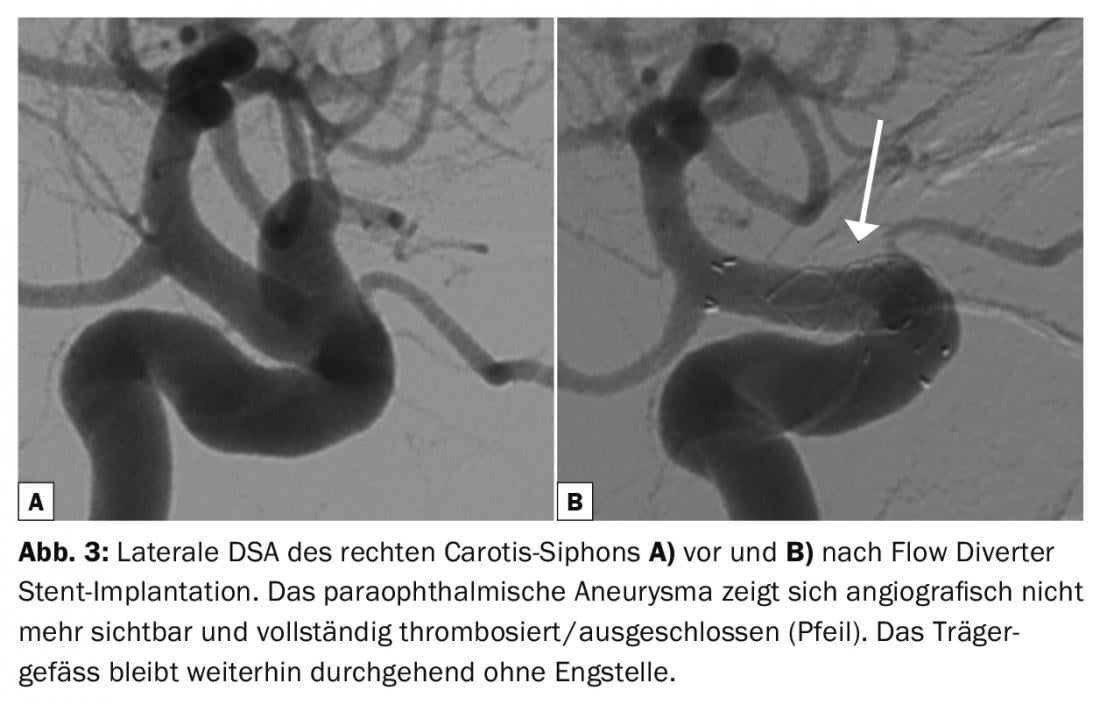
Extra-saccular implants: The introduction of low-profile, flexible, self-expanding stents that can be implanted through microcatheters or dual-lumen balloons are a breakthrough in the treatment of distal or complex aneurysms [17]. The latest generation of tightly braided stents, called flow diverters, redirect blood flow to induce thrombosis of the aneurysm without filling the aneurysm sac with implants (Fig. 3). Sometimes additional coiling of giant aneurysms is recommended to rule out delayed rupture [18]. New neck-bridging devices that protect the aneurysm-bearing vessel and efferent vessel and preserve blood flow during coiling of broad-based bifurcation aneurysms are currently being evaluated (Comaneci, Pulse Rider, eCLIP, pCONus) [19–22]. The PCANvas [23] is an experimental device similar to the pCONus that contains an additional membrane to increase flow interruption. Clinical investigations are currently underway.
Hybrid interventions
Some operating rooms provide facilities and materials for combined surgical and endovascular procedures to allow both neurovascular disciplines to collaborate directly.
Hybrid interventions not only have an impact on surgical management, but can also help increase cost efficiency.
Limitations
Despite continued medical advances, treating some aneurysms is a major challenge – sometimes an impossibility, no matter which technique is chosen. Specifically, giant fusiform basilar artery aneurysms have a very poor spontaneous course with high mortality and morbidity because of their difficult-to-control growth. This is due to hemorrhages, the space-occupying effect and the repeated strokes of the brain stem [24]. Many endovascular and microsurgical strategies have been tried, some with success, but they all imply high treatment risk.
Conclusion
During the last two decades, many innovative surgical and endovascular techniques have been developed. Although new endovascular developments are in focus, a relevant number of aneurysms require highly specialized surgical therapy. Interdisciplinary meetings are the cornerstone of optimal patient care, regardless of which treatment method is being considered.
Take-Home Messages
- New titanium aneurysm clips ensure high closing force and cause hardly any MRI artifacts.
- Vascular reconstructive surgery for complex aneurysms, utilizing multiple and different types of aneurysm clips, is a component of modern vascular centers.
- The WEB device is the latest intrasaccular device. Its nitinol cage of self-expanding tight mesh promotes intrasaccular thrombosis.
- Flexible, self-expanding stents are a breakthrough in the treatment of distal or complex aneurysms. So-called flow diverters redirect blood flow to cause thrombosis of the aneurysm.
Literature:
- Zada G, et al: Neurosurg Focus 2009; 26: E7.
- Guglielmi G, et al: J Neurosurg 1991; 75: 8-14.
- Moret J, et al: Interv Neuroradiol 1997; 3: 21-35.
- Lanzino G, et al: J Neurosurg 1999; 91: 538-46.
- Hopf NJ, et al: Minim Invasive Neurosurg 2009; 52: 126-31.
- Horiuchi T, et al: Neurosurg Rev 2013; 36: 133-7.
- Acciarri N, et al: J Neurosurg Sci 2016; 60: 83-94.
- Yang I, et al: Neurosurgery 2008; 62: ONS371-8; discussion 8-9.
- Tayebi Meybodi A, et al: J Neurosurg 2016; 4: 1-17.
- Raabe A et al: Neurosurgery 2003; 52(1): 132-9; discussion 139.
- Raabe A et al: J Neurosurg 2005; 103(6): 982-9.
- Lane B, et al: J Neurosurg 2015; 122: 618-26.
- Staarmann B, et al: World Neurosurg 2017; 100: 522-30.
- Broeders JA, et al: J Neurointerv Surg 2016; 8: 898-908.
- Sourour NA, et al: Neurosurgery 2017 (epub, ahead of print).
- Pierot L, et al: AJNR Am J Neuroradiol 2017; 38: 1151-5.
- Akmangit I, et al: AJNR Am J Neuroradiol 2015; 36: 323-9.
- Rouchaud A, et al: Neuroradiology 2016; 58: 171-7.
- Fischer S, et al: J Neurointerv Surg 2016; 0: 1-5.
- Spiotta AM, et al: J Neurointerv Surg 2016; 8: 186-9.
- Chiu AH, et al: J NeuroSurg 2017; 17: 1-8.
- Perez MA, et al: J Neurointerv Surg 2017; 9: 39-44.
- Perez MA, et al: J Neurointerv Surg 2016; 8: 1197-201.
- Saliou G, et al: Stroke 2015; 46: 948-53.
InFo NEUROLOGY & PSYCHIATRY 2017; 15(5): 8-10.