At the ESMO Congress in Amsterdam, speakers from various countries discussed the latest results and therapy recommendations in the field of chronic lymphocytic leukemia. Overall, chemoimmunotherapy with fludarabine, cyclophosphamide (FC), and the CD20 antibody rituximab (R) shows good efficacy, but the treatment regimens must be more targeted in the future and especially applicable to recurrent forms, the experts concluded.
According to Prof. Paolo Ghia, MD, Milan, new insights into genetics in chronic lymphocytic leukemia (CLL) have been made in recent years: “Studies emphasize, for example, the relevance of pre-mRNA splicing, a critical cellular process that may contribute to chronic lymphocytic leukemia [1, 2]. Furthermore, periodically occurring NOTCH1, MYD88, and XPO1 mutations promote the clinical development of the disease [3].” Ultimately, however, the influence of some mutations appears to disappear when therapy is provided by allogeneic hematopoietic stem cell transplantation [4].

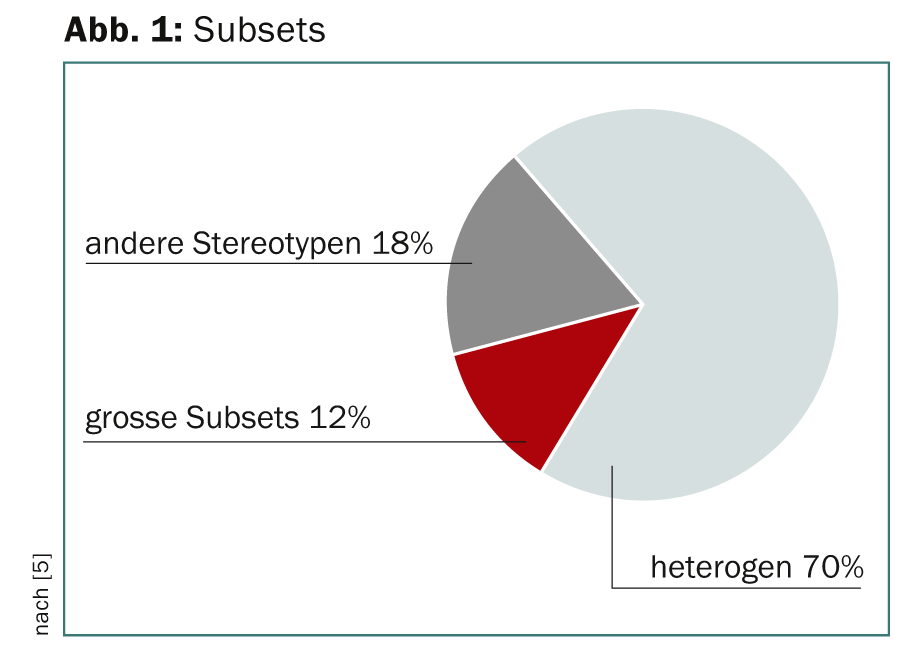
Functionally and prognostically relevant is the assumption that CLL can be divided into distinct subsets with stereotyped B-cell receptors (BCRs). Thus, molecular classification and targeted therapeutic interventions applicable to a large number of patients in the same subset would be possible. Sequence analysis revealed two superordinate groups for CLL patients: One with stereotyped, one with non-stereotyped BCR, with a ratio of 1:2 [5]. 12% of the stereotype group formed large subsets (Fig. 1).
First-line treatment
Peter Hillmen, MD, Leeds, presented ways of first-line therapy of CLL: “Randomized phase III trials [6] conclude that chemoimmune therapy with fludarabine, cyclophosphamide, and rituximab (FCR) improves progression-free and overall survival. Three years after randomization (the second group underwent chemotherapy with fludarabine, cyclophosphamide), 65% of the first group was progression-free, compared with only 45% in the second. The authors conclude that specific first-line therapy can alter the natural history of CLL. These results defined the new standard of care in CLL treatment.” If any of the following criteria apply to a patient, full-dose combination (FCR) is not recommended:
- Age: 75 years or older
- WHO performance status 2 or 3
- Cardiac limitations (NYHA class II), respiratory (bronchiectasis or moderate COPD), or renal problems.
Other therapy options
The controversial question is whether fludarabine is more effective than chlorambucil as monotherapy (first-line) in patients older than 65 years. Although fludarabine achieves significantly higher remission rates, neither progression-free nor overall survival is improved compared with chlorambucil [7].
Ofatumumab, a human monoclonal antibody (anti-CD20), shows good efficacy and tolerance in monotherapy in patients with fludarabine insensitivity, including those previously treated with rituximab [8]. “To date, comparative studies comparing ofatumumab with rituximab are lacking. What is suspected is that combining ofatumumab with chlorambucil may improve progression-free survival compared with chlorambucil monotherapy,” Dr. Hillmen said.
Furthermore, a new type II, anti-CD20 antibody called obinutuzumab (GA-101) seems to offer a promising approach for a new therapy [9].
Current therapy not effective enough?
Despite the many options: Dr. Hillmen concludes that current treatment pathways are ineffective for CLL. This is for several reasons:
Toxicity: First, damage occurs to normal cells. In addition, CLL cells are increasingly developing resistance. “Current therapies are not targeted,” Dr. Hillmen emphasized.
Efficacy: Only a small number of patients achieve true, complete remission. Relapses are therefore inevitable and the majority eventually die of CLL.
New approaches that seek to better understand the pathophysiology are thus urgently needed, for example, by means of the tyrosine kinase inhibitor ibrutinib, which in initial phase Ib/II multicenter trials enabled sustained remission rates in patients with recurrent or persistent CLL, including those with high-risk genetic lesions [10].
Recurrences CLL
In recurrent CLL, allogeneic hematopoietic stem cell transplantation is the gold standard because it leads to good disease control independent of mutations [4].
Prof. Dr. med. Stephan Stilgenbauer, Ulm, Germany, described the optimal treatment pathway as follows: “Relapsed CLL is a biologically and clinically heterogeneous disease that should initially be approached with a repeat of the initial treatment regimen after a remission of >24 (-36) months. Standards here are FCR, R-bendamustine, R-chlorambucil therapies. Unfortunately, a satisfactory standard of care for relapse at 24-36 months is otherwise lacking. Allogeneic hematopoietic stem cell transplantation is an option for young high-risk patients. New biological agents (BCR signaling antagonists, BCL2 inhibitors, type II antibodies, etc.) and future chemotherapy-free or at least -reduced combinations offer hope.”
Source: “Treatment of Chronic lymphocytic Leukaemia,” Session at the 38th ESMO Congress, September 27-October 1, 2013, Amsterdam.
Literature:
- Wang L, et al: N Engl J Med 2011; 365: 2497-2506. doi: 10.1056/NEJMoa1109016.
- Quesada V, et al: Nat Genet 2011 Dec 11; 44(1): 47-52. doi: 10.1038/ng.1032.
- Puente XS, et al: Nature 2011 Jun 5; 475(7354): 101-105. doi: 10.1038/nature10113.
- Dreger P, et al: Blood 2013 Apr 18; 121(16): 3284-3288. doi: 10.1182/blood-2012-11-469627. epub 2013 Feb 22.
- Agathangelidis A, et al: Blood 2012; 119(19): 4467-4475.
- Hallek M, et al: The Lancet 2010; 376(9747): 1164-1174.
- Eichhorst BF, et al: Blood 2009 Oct 15; 114(16): 3382-3391. doi: 10.1182/blood-2009-02-206185. epub 2009 Jul 15.
- Wierda WG, et al: Published online before print Blood 2011. doi: 10.1182/blood-2011-04-348656.
- Mössner E, et al: Blood 2010 Jun 3; 115(22): 4393-4402. doi: 10.1182/blood-2009-06-225979.
- Byrd JC, et al: N Engl J Med 2013; 369: 32-42. doi: 10.1056/NEJMoa1215637.
InFo Oncology & Hematology 2013; 1(1): 39-40.
CongressSpecial 2014; 6(1): 19-20











