Polycystic ovary syndrome (PCO-S) is among the most common causes of infertility/infertility and is one of the most common endocrinologic disorders in women, accounting for 5% to 10% [1,2]. Currently, the most commonly used definition (Rotterdam criteria) includes two of the following three symptoms: polycystic ovaries – oligo-/amenorrhea, and/or clinical signs of hyperandrogenemia. About 50% of those affected are obese. In these women in particular, insulin resistance can usually be detected, and the transition to metabolic syndrome is smooth. In lean PCO-S patients, hyperandrogenemia and/or anovulation are prominent. The therapy of PCO-S depends on the symptoms that are in the foreground.
The disease occurs in various forms, from mild, asymptomatic PCO-S to the extreme form, Stein-Leventhal syndrome. Affected patients usually report to the clinic for two reasons: because of an unfulfilled desire to have children (up to 74%) or because of cycle disorders, mostly oligo- to amenorrhea (up to 50%), which usually occur during puberty.
In addition to the most common symptoms, however, also show:
- Signs of increasing androgenization
- Laboratory changes with increased LH and normal or decreased FSH levels.
- Increased androgen levels
- In ultrasound analysis, the typical picture of enlarged ovaries with numerous small follicles arranged subcortically like a string of pearls (necklace sign).
These clinical signs do not all have to occur at the same time. Individual symptoms can also already indicate a disease.
Whereas PCO-S was initially regarded primarily as a syndrome characterized by androgenization manifestations and unfulfilled desire to have children, today the metabolic syndrome associated with PCO-S with its life-threatening long-term consequences is coming to the fore [3]. Therefore, early diagnosis and therapy are of great importance to reduce the later increased risk of cardiovascular disease, type 2 diabetes and endometrial cancer.
Because of its clinical significance, PCO-S not only concerns gynecologists and dermatologists, but increasingly also internists. The exact causes of PCO-S are still unclear. Given familial clustering and results from twin studies, genetic causes are thought to play a role. Indeed, several gene loci have been identified that are thought to be associated with the development of PCOS [4].
Pathogenesis of PCO-S
The female androgens are produced in varying proportions in the adrenal cortex and ovaries. Testosterone production in the adrenal cortex and ovaries is regulated by releasing hormones from the pituitary gland. Cortisol and estradiol cause negative feedback by inhibiting the release of releasing hormones (Fig. 1).
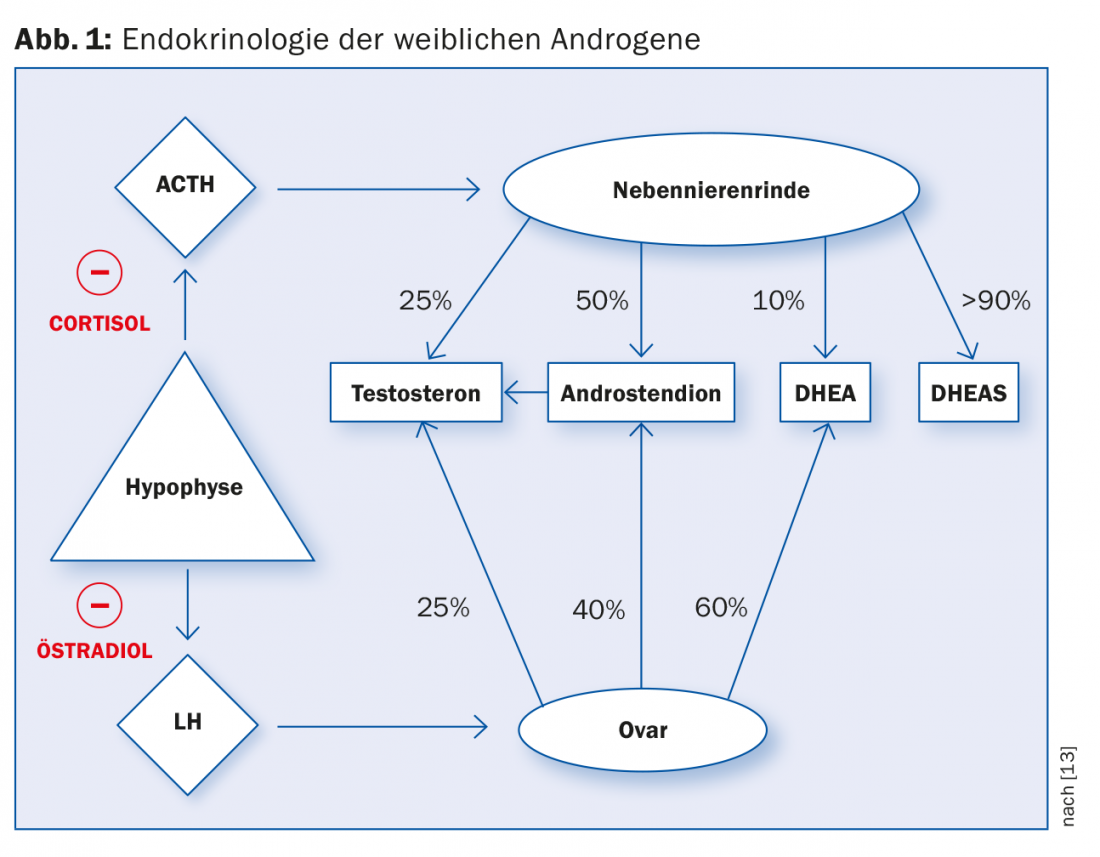
Basically, it is assumed that PCO-S is due to a disturbance of the endocrine hypothalamic-pituitary-ovarian regulatory circuit. Probably there is a lack of activity of aromatases in the granulosa cells of the ovary, which are stimulated by FSH in healthy women. Hyaline thickening of the basement membrane leads to inhibition of FSH action, whereupon granulosa cells degenerate due to inadequate stimulation. At the same time, steroid biosynthesis in the ovary is derailed due to continuous gonadotropic stimulation by LH, whereupon more androgens are produced. Subclinical hypothyroidism is found in 10 to 25% of patients. The connection and influence on the pituitary-ovarian regulatory circuit is still unclear [5].
Diagnostic measures
In addition to the most common symptoms of sterility and oligo- resp. Amenorrhea, is part of confirming the diagnosis (see also Fig. 2):
- An extensive history regarding menarche, menstrual cycle, family history.
- A clinical examination with special attention to increasing androgenization with hirsutism up to 70%, acne up to 35%, alopecia, obesity up to 85% (BMI).
- Hormonal assessment in the early follicular phase, fasting with determination of: LH, FSH, prolactin, estrone (E1), estradiol (E2), SHBG (sex hormone binding globulin), DHEA (dehydroepiandrosterone), DHEA-S (dehydroepiandrosterone sulfate), 17-alpha-OH-progesterone, cortisol, TSH, testosterone, androstenedione.
- Transvaginal ultrasonography showing enlarged ovaries with numerous small follicles.
- If metabolic syndrome is suspected, insulin resistance must also be investigated. Fasting glucose, fasting insulin, calculation of homeostasis model assessment (HOMA) score, determination of LDL, HDL and triglycerides, blood pressure.
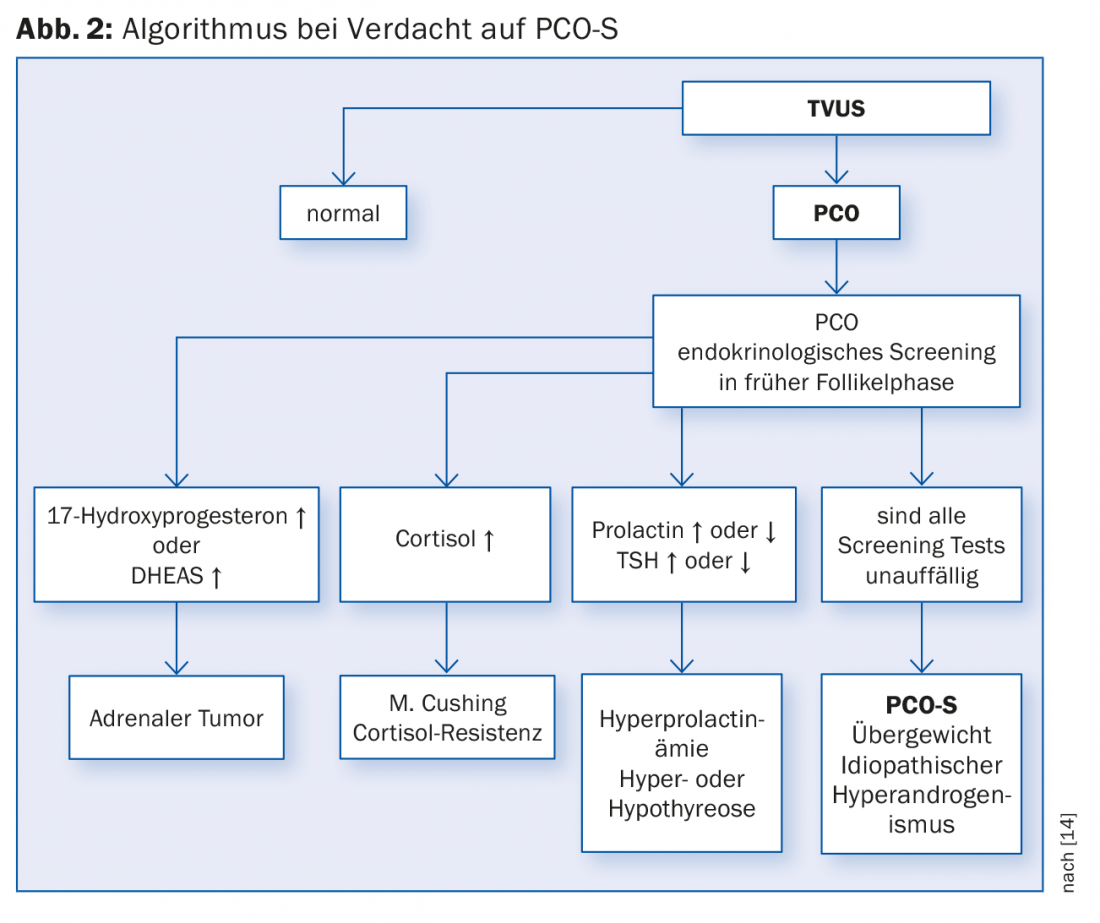
ad 1) About 80% of patients report menstrual disorders, mostly oligo- to amenorrhea each up to 50%, which mostly occur during puberty. It is important to ask about the age at menarche as well as the subsequent cycle pattern, as hyperandrogenemia often leads to chronic anovulation or secondary amenorrhea early on. Also, ask about other family members who have had acne or hirsutism.
ad 2) On clinical examination, hirsutism is recorded using the Ferriman and Gallwey score [6].
ad 3) Hormonal evaluation reveals elevated LH and normal or decreased FSH levels. This is because LH is released pulsatile from the anterior pituitary in response to the pulsatile release of GnRH (gonadotropin-releasing hormone) from the hypothalamus. The androgenic androstenedione, testosterone and, somewhat less frequently, DHEA are also elevated. Similarly, estrone (E1) levels are elevated compared to estradiol (E2) levels in the early follicular phase [7]. In women with hirsutism, free testosterone should be measured first. If the presence of an adrenal tumor is suspected, DHEA and DHEA-S should also be measured.
Typical hormonal findings in PCO-S:
- Borderline high or elevated androgens (testosterone, androstenedione, DHEAS)
- Elevated LH/FSH quotient, (i.e. FSH in normal range) > 1.0 early-cycle
- Decreased SHBG
ad 4) These clinical signs need not all occur simultaneously. The appearance of individual symptoms may already indicate a disease. Because of the heterogeneity of this clinical picture, it is sometimes difficult to make a definite diagnosis. Therefore, different groups have come up with different diagnostic criteria. In 2012, a National Institute of Health (NIH) workshop analyzed the different diagnostic criteria and recommended that the diagnosis of PCO-S be made according to the Rotterdam criteria [8]. The Rotterdam criteria are the result of an international consensus meeting held in Rotterdam in 2003 [9], see Table 1.

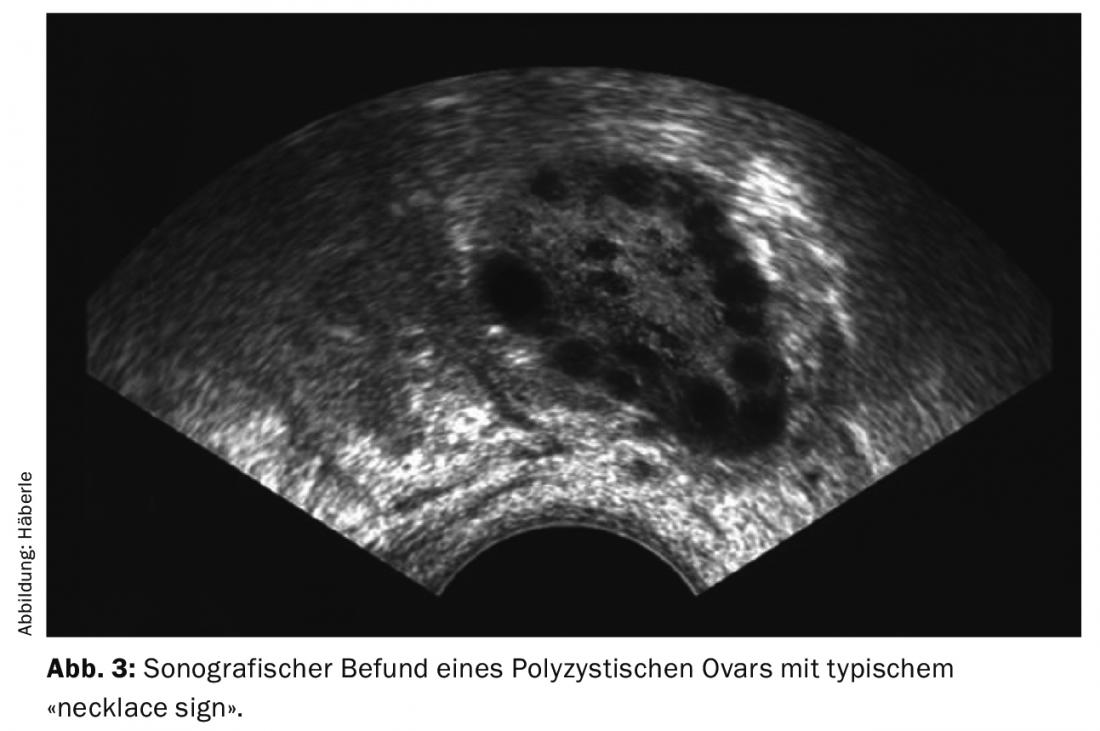
The diagnosis of polycystic ovaries (PCO) is made sonographically (preferably by vaginal sonography) (Fig. 3). According to the current definition (Rotterdam criteria), 12 or more follicles of 2-9 mm per ovary and/or an ovarian size of >10 ml (ovarian volume = 0.5 × length × width × thickness) are considered polycystic. For diagnosis, it is sufficient if one ovary fulfills the criteria. When taking ovulation inhibitors, the ovaries cannot be assessed in this regard.
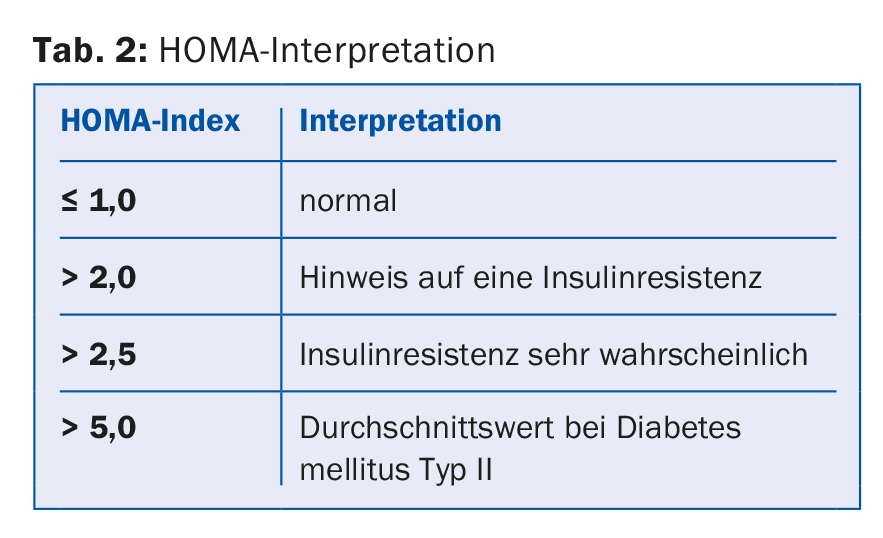
ad 5) If metabolic syndrome is suspected, insulin resistance must also be clarified. For this purpose, fasting glucose and fasting insulin are determined and from this the HOMA index is determined [10] (Tab. 2).
Formula for determining the HOMA index:
HOMA index = insulin (fasting, µU/ml) × blood glucose (fasting, mg/dl) / 405
HOMA index = insulin (fasting, µU/ml) × blood glucose (fasting, mmol/l) / 22.5
Instead of determining the HOMA index, an oral glucose tolerance test (oGTT) with 75 g glucose can also be performed. The metabolic syndrome in obese women also includes elevated LDL levels combined with low HDL and triglyceride levels.
It is under discussion whether the anti-Müllerian hormone (AMH) concentration in serum should also be determined to confirm the diagnosis. But the data to date are inconclusive, and therefore AMH determination is not yet recommended for routine use [11]. The final diagnosis of PCO-S is made after other differential diagnoses such as primary hypothyroidism, hyperprolactinemia, adrenal hyperplasia, androgen-producing tumors, adrenogenital syndrome (AGS), and Cushing’s disease have been excluded. It is also important to exclude exogenous androgen supply.
Long-term health consequences of PCO-S
Women with PCO-S diagnosed according to these criteria are at increased risk for the following concomitant diseases: The irregular cycles due to anovulation are mostly caused by the lack of progesterone, the “counterpart” of estrogen, which leads to an unfulfilled desire to have children. Obesity leads to an increased risk of developing metabolic syndrome, type 2 diabetes, also as a result of a positive family history of type 2 diabetes and defective insulin activity (insulin resistance and beta cell dysfunction). Therefore, the risk of arterial hypertension and cardiovascular disease also increases. The combination of obesity and hyperandrogenism results in elevated estrogen levels due to aromatization of androgens in adipose tissue. This increases the risk of developing endometrial cancer.
Therapy
Not only biochemically, but also clinically, PCO-S shows itself to be very heterogeneous.
Treatment depends primarily on what is most important to the patient. In addition, the long-term goal is to avoid the concomitant diseases.
Sterility: The aim of therapy is ovulation induction by means of hormonal stimulation. Administration of the antiestrogen clomiphene (must be ordered in Switzerland through an international pharmacy) is the first-line therapy. Patients who demonstrate hyperprolactinemia should be treated with bromocriptine or cabegoline. Glucocorticoids should be given to patients with an adrenal component with elevated DHEA-S levels before stimulating with clomiphene. Gonadotropins such as human menopausal gonadotropin (HMG) or FSH should be used only in cases of resistance to therapy. In such cases, it is sometimes also helpful to pretreat with a GnRH agonist prior to gonadotropin therapy, which increases the success of therapy [12]. This form of therapy should only be performed by experienced clinicians who also carefully monitor follicular growth with ultrasound. This is because in PCO-S patients, a small dosage is sometimes sufficient to stimulate the many follicles to grow, despite spontaneous absence of ovulation. This can lead to hyperstimulation syndrome and consecutive higher-order multiple pregnancies.
A surgical procedure such as bilateral ovarian wedge resection or destruction of ovarian tissue with electrocoagulation should only be undertaken as “ultima ratio”. It has not yet been proven that a regular cycle occurs after such therapy. In addition, adhesions can occur as a result of such operations, which can be a hindrance to conception. After unsuccessful conservative therapy over six months with hormonal stimulation with or without insemination, IVF/ICSI is indicated.
Obesity and metabolic syndrome with insulin resistance: The first therapeutic measure is BMI reduction through lifestyle modification with regular physical activity, supported by nutritional counseling. If these measures do not lead to success, bariatric surgery can lead to the goal. Weight loss corrects virtually all pathological parameters in PCO-S and thus can lead to an ovulatory cycle. Even a weight reduction of 5 to 10% improves hirsutism in about 50% of patients within six months.
Metformin (2 × 500 mg up to 3 × 850 mg metformin/day, max. to 2500 mg/day), a biguanide derivative and antidiabetic agent, improves the chance of restoring normal ovulatory cycles by reducing ovarian androgen secretion. Metformin can be combined with ovulation inhibitors without any problems. It is being used increasingly generously in all forms of PCO-S.
Acne and hirsutism: Cyclic, antiandrogens (e.g., cyproterone acetate) as progestogens, oral contraceptives that cause decreased ovarian androgen secretion on the one hand and increased peripheral SHBG concentrations on the other, are helpful here. This often results in decreased peripheral androgen activity. In about 60% of patients, acne is no longer detectable after six months of treatment. Glucocorticoids are used in patients with increased adrenal androgen production.
Literature:
- Azziz R, Woods KS, Reyna R, et al: The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004; 89(6): 2745.
- Ehrmann DA: Polycystic ovary syndrome. N Engl J Med 2005 March 24; 352(12): 1223-36.
- American Association of Clinical Endocrinologists Polycystic Ovary Syndrome Writing Committee. American Association of Clinical Endocrinologists Position statement on metabolic and cardiovascular consequences of polycystic ovary syndrome. Endocr Pract. 2005 Mar-Apr; 11(2): 126-34.
- World CK, Duran JM: Genetics of polycystic ovary syndrome. Semin Reprod Med. 2014 May;32(3): 177-82.
- Pergialiotis V, et al: Management of endocrine disease: The impact of subclinical hypothyroidism on anthropometric characteristics, lipid, glucose and hormonal profile of PCOS patients: a systematic review and meta-analysis. Eur J Endocrinol. 2017 Mar;176(3): R159-R166.
- Hatch R, Rosenfield RL, et al: Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981 Aug 1;140(7): 815-30.
- Rebar R, Judd HL, et al: Characterization of the inappropriate gonadotropin secretion in polycystic ovary syndrome. J Clin Invest. 1976 May;57(5):1320-9.
- NIH. Polycystic Ovary Syndrome (PCOS) – Resources. http://prevention.nih.gov/workshops/2012/pcos/resources.aspx (Accessed on March 19, 2013).
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004;19: 41.
- Carmina E, Lobo RA: Use of fasting blood to assess the prevalence of insulin resistance in women with polycystic ovary syndrome. Fertile Sterile. 2004 Sep; 82(3): 661-5.
- Eilertsen TB, Vanky, et al: Anti-Mullerian hormone in the diagnosis of polycystic ovary syndrome: can morphologic description be replaced? Hum Reprod. 2012 Aug; 27(8): 2494-502.
- Filicori M, Valdiserri A, et al: Ovulation induction with pulsatile gonadotropin-releasing hormone: Technical modalities and clinical perspectives. Fertil Steril 56: 1, 1991.
- From Burger HG: Fertil Steril. 2002; 77 Suppl 4): S3-S5 and Simon JA: Fertil Steril.2002; 77(Suppl 4): 77-82) and Brand JS et al: Int J Impot Res. 2010; 22(2): 91-104.
- From Buggs C, Rosenfield RL: Polycystic ovary syndrome in adolescence. Endocrinol Metab Clin North Am 2005; 34: 677.
HAUSARZT PRAXIS 2017; 12(3): 28-32











