Drug treatments are often associated with additional risks in elderly patients due to polypharmacotherapy and age-related changes in metabolism, especially since the efficacy and tolerability of drugs in the elderly are often poorly studied. Herbal medicines are largely unproblematic and well tolerated in old age and thus offer themselves as alternatives to synthetics. For anxiety disorders, Silexan is a potent herbal anxiolytic with proven efficacy in the elderly that is non-sedating and does not produce dependence or withdrawal effects. WS 5570 is as effective in depressed patients 60 years of age and older as it is in younger patients. The adverse effects observed in studies are at placebo level. However, possible interactions, especially with immunosuppressants and cytostatic drugs, should be considered.
According to estimates by the Swiss Federal Statistical Office in March 2011, the proportion of people aged 65 and over will rise from 17% in 2010 to 26% in 2035. The authors forecast a doubling of the proportion of older people in six cantons and a proportion of over 30% in three cantons. Thanks to improved health education measures, medical prophylaxis and therapeutic options, increases in life expectancy and a comparable rise in the proportion of older people in the population can also be observed in the other western industrialized nations. Thus, not only the typical “diseases of old age” represent an increasing challenge for medicine, but also, in general, the adequate care of an increasingly aging, often multimorbid patient population.
With increasing age, functional limitations and a deterioration of physiological regulatory mechanisms are associated with pharmacokinetic and pharmacodynamic changes relevant to drug intake, such as a reduction in hepatic and renal clearance. In addition, the multimorbidity of many elderly patients necessitates polypharmacotherapy, which often causes incalculable risks, especially in the outpatient setting, such as an increase in the risk of falls, delirium, bleeding complications, cognitive impairment, or increased mortality.
In contrast to synthetic drugs, phytopharmaceuticals are complex multisubstance mixtures with a comparatively broad action profile. Side effects due to mechanism of action, especially those with severe manifestations, are mostly rare or even unknown; the interaction potential is low for many herbal preparations compared to synthetic therapeutics [1]. With proven efficacy, they therefore offer an alternative to synthetic therapeutics, especially for older people with often chronic diseases. Because of their favorable ratio of efficacy and tolerability, they improve the quality of life of those affected. This applies not least to the affective disorders that are particularly common in old age. Here we report, by way of example, the efficacy of treating anxiety disorders with Silexan and depression with the St. John’s wort extract WS 5570 in elderly patients. A more detailed version of the data analysis and discussion of the same was also published in Wiener Medizinische Wochenschrift [2].
Silexan for anxiety disorders
Silexan is the active ingredient in the product Lasea® (Dr. Willmar Schwabe GmbH & Co. KG, Karlsruhe, Germany), which is registered in Germany. The patented active ingredient from flowers of Lavandula angustifolia is available in capsules for oral administration and is approved in Germany as a drug in a daily dose of 1× 80 mg for the treatment of restlessness in anxious mood. Thus, a herbal anxiolytic with proven efficacy [3] is available.
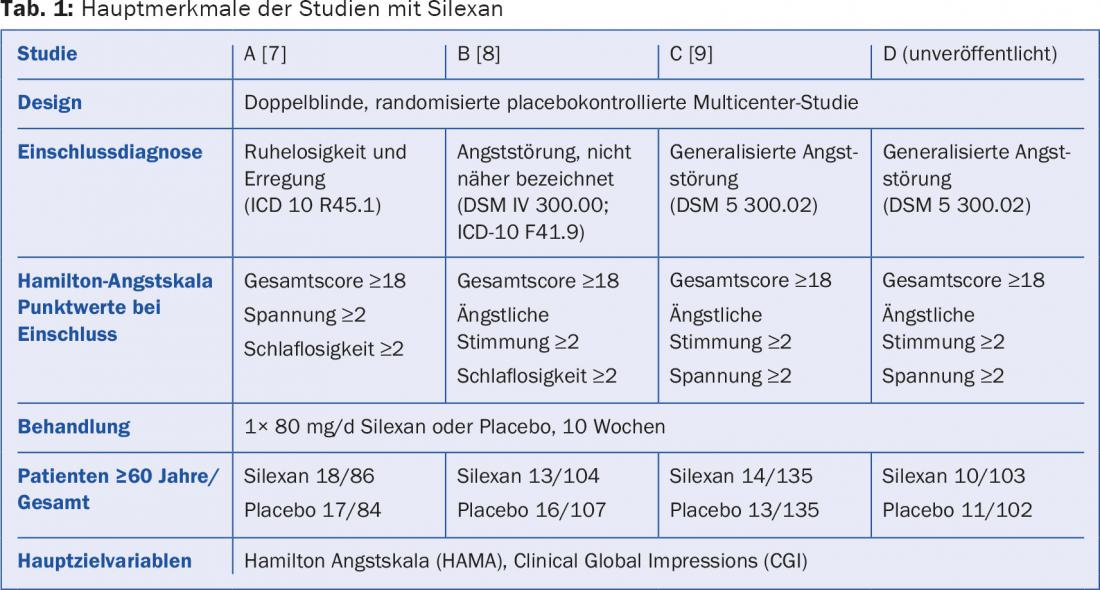
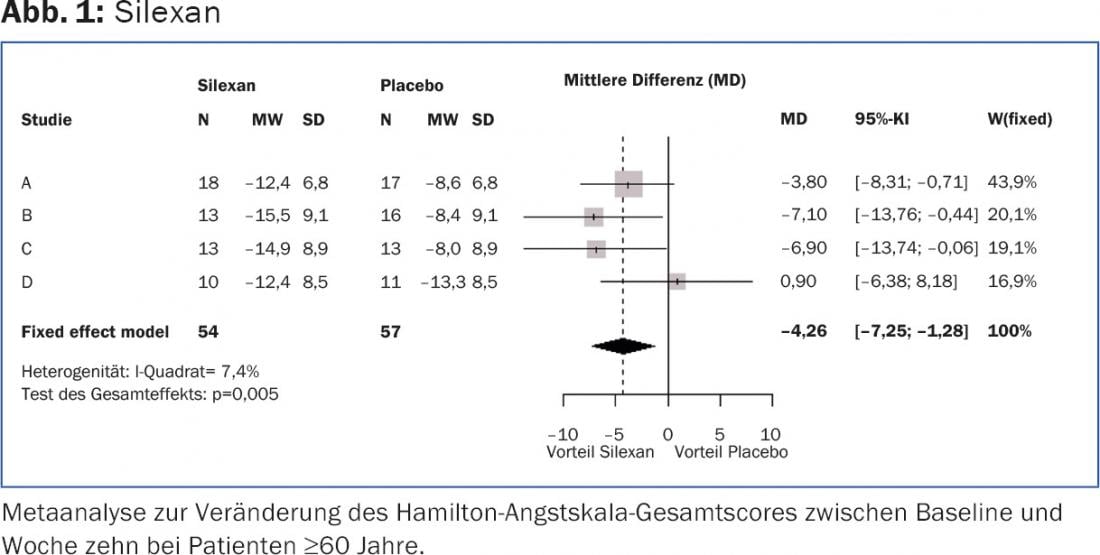
A meta-analysis of four clinical trials (Table 1) involving a total of 857 adult anxiety patients taking either 80 mg silexan or placebo evaluated data from the subgroup of 112 participants aged 60 years and older. In this population, the Hamilton Anxiety Scale (HAMA) total score decreased significantly more over the course of treatment with Silexan than with placebo in three of the four primary studies. In the pooled data set of all studies, the score decreased under Silexan from 25.1 ± 5.7 (mean ± S) points at baseline to 11.6 ± 7.4 points at end of treatment, compared with a decrease from 25.2 ± 5.8 points to 15.7 ± 7.6 points under placebo. In the meta-analysis, there was a statistically significant mean difference of 4.3 points in favor of silexan (p<0.01; Fig. 1).
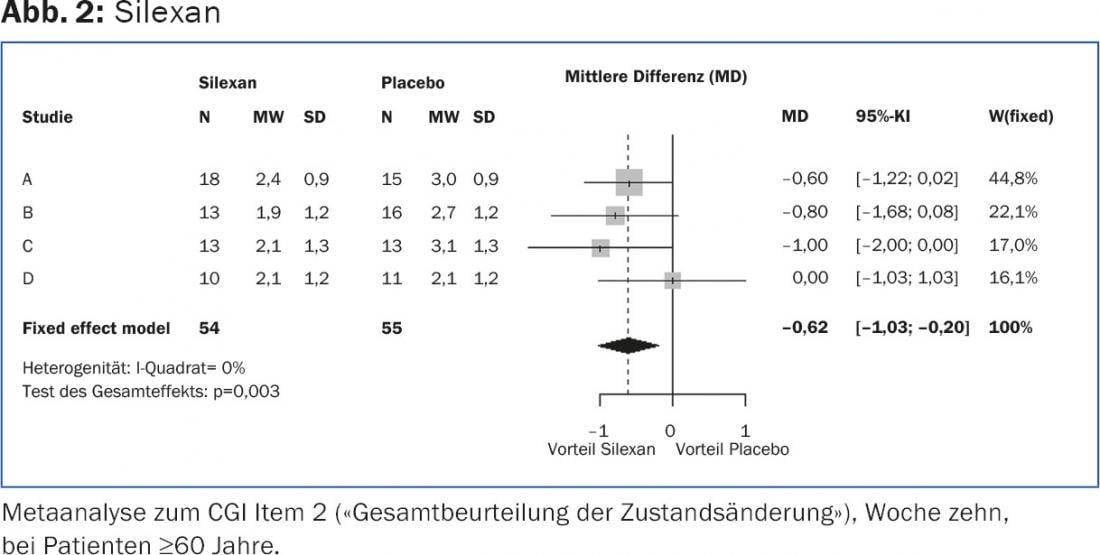
In the overall picture of those over 60 years of age, mean disease severity (CGI item 1) before treatment initiation ranged from four (“moderately ill”) to five points (“markedly ill”) in all primary studies, with mean values of 4.5 ± 0.6 points each for Silexan and placebo across all studies. In three of the four primary studies, Silexan showed a significantly greater improvement in global judgment over the course of therapy than placebo, with mean scores decreasing to 3.0 ± 1.1 points (3 points: “mildly ill”) in Silexan-treated patients across all studies and to 3.4 ± 1.3 points in the placebo group. For the change in overall clinical picture (CGI item. 2), mean scores at the end of treatment in the pooled data set were 2.1 ± 1.0 points for silexan and 2.8 ± 1.3 points for placebo (2 points: “much better”; 3 points: “only slightly better”). The group difference was significant in the meta-analysis in favor of Silexan (p<0.01; Fig. 2).
The results demonstrate significant benefits of Silexan over placebo in the age group of patients 60 years and older, both in terms of anxiolysis and in the overall psychiatric picture.
WS 5570 for depressive disorders
WS 5570 is the active ingredient of the products Hyperiplant® (Switzerland) and Neuroplant® (Germany; Dr. Wilmar Schwabe GmbH & Co. KG, Karlsruhe). This is a stabilized dry extract of St. John’s wort (Hypericum perforatum) whose efficacy in depressive disorders has been demonstrated in numerous clinical trials [4]. To evaluate the efficacy of WS 5570 specifically in elderly depressed patients, data were pooled from four controlled trials of acute treatment of mild, moderate, or severe depressive episodes, and daily doses of the extract ranging from 600 to 1800 mg compared with placebo or paroxetine. (Tab. 2). Project H was a long-term study whose upstream single-blind acute phase was included in the evaluation.
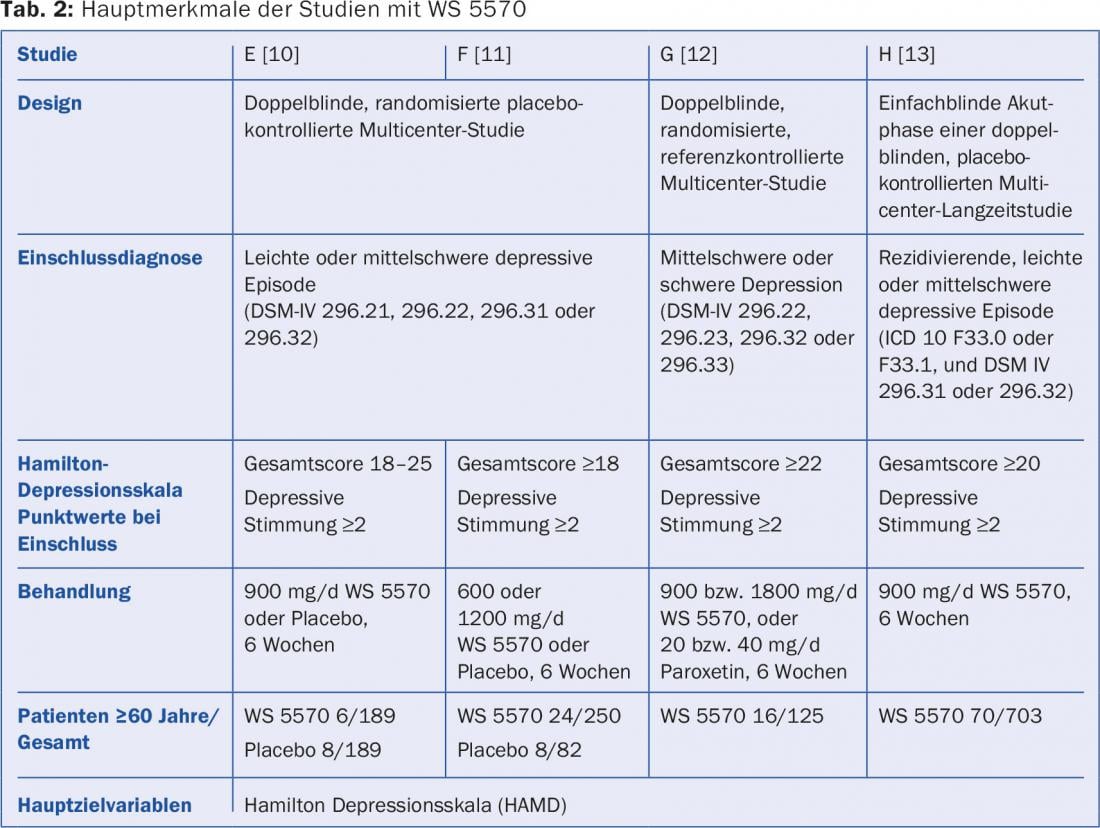
116 of the patients treated with WS 5570 in the four trials were at least 60 years old. The comparison groups were the patients under 60 years of age also treated with WS 5570 (n=942) and the patients in the placebo groups of studies E and F (n=270; 16 patients ≥60 years).
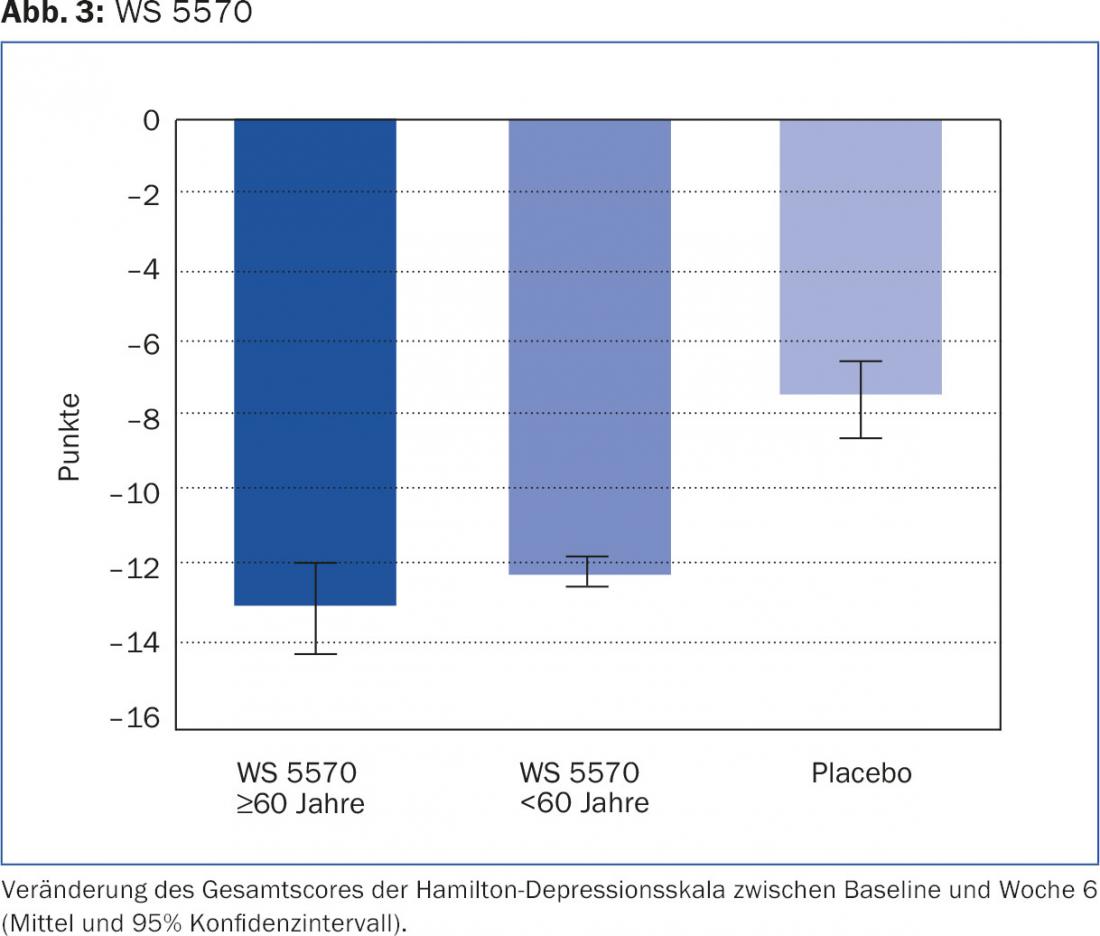
The severity of depression was primarily assessed using the Hamilton Depression Scale (HAMD) in all studies. Before treatment initiation, the mean HAMD total scores of all compared groups were homogeneously in the range of 22.4 ± 2.8 to 23.5 ± 3.1 points. Between baseline and the end of the six-week acute phase, the score decreased by 13.2 ± 6.0 points in patients ≥60 years of age treated with WS 5570, and in patients treated with WS 5570 <60 years by 12.3 ± 6.6 points and in patients in the placebo groups by 7.5 ± 7.4 points. (Fig.3).
Patients with a HAMD total score decrease of ≥50% of baseline with acute treatment were classified as responders; a final score ≤7 points as an indicator of remission. Responder and remission rates were 75.9% and 27.6% in patients 60 years of age and older treated with WS 5570, 70.9% and 31.9% in younger patients treated with WS 5570, and 39.3% and 19.3% on placebo.
Overall, the results for WS 5570 show as pronounced an antidepressant effect in those ≥60 years of age as in younger patients. In both age groups, the efficacy of WS 5570 was significantly superior to that of placebo.
Compatibility
The adverse effects of Silexan are limited to gastrointestinal discomfort (primarily belching) and allergic skin reactions. Unlike other drugs administered as anxiolytics, such as selective serotonin reuptake inhibitors (SSRIs) or benzodiazepines, Silexan is not known to have sedative effects or discontinuation or dependence effects. Interactions with other drugs have also not been described to date [5].
In clinical trials, WS 5570 was found to be better tolerated compared to synthetic antidepressants (among others in [6]). Effects such as sedation and anticholinergic reactions were not more frequent under treatment with Hypericum extract than under placebo. In rare cases, gastrointestinal complaints, allergic reactions and fatigue or restlessness may occur during treatment with WS 5570. Very rarely, sunburn-like reactions may occur due to photosensitization. St. John’s wort-containing drugs may promote the metabolism or transport of drugs metabolized via cytochrome P450-3A4, -2C9, -2C19, or p-glycoprotein.
Conclusions
Especially in elderly patients, limitations in daily living skills are often accompanied by affective disorders. Unfortunately, the adverse effects of many medications prescribed for anxiety disorders and depression (e.g., sedation, weight gain, sexual dysfunction, cognitive impairment) contribute to further reducing the daily activities and sense of well-being of affected patients, thus worsening the symptomatology they were actually prescribed to treat. None of the above adverse effects were observed for Silexan or WS 5570.
The analyses presented here show that in the indications of anxiety and depression, herbal treatment alternatives exist for the general practitioner, whose efficacy in elderly patients has been proven by clinical trials and which exhibit significantly fewer adverse effects than comparably effective synthetic therapeutics. Silexan and WS 5570 thus offer themselves as effective and well-tolerated therapeutic options for the treatment of affective disorders also in geriatric patient populations.
Literature:
- Loew D: Ars Medici Theme Phytotherapy 2012; 1: 15-20.
- Kasper S: Wien Med Wochenschr 2015 (in press).
- Kasper S: Int J Psychiatry Clin Pract 2013; 17(Suppl 1): 15-22.
- Kasper S, et al: Psychoneuro 2006; 32(10): 494-498.
- Kasper S, et al: Z Phytother 2011; 32(2): 60-63.
- Kasper S, et al: Int Clin Psychopharmacol 2010; 25(4): 204-213.
- Kasper S, Anghelescu I, Dienel A: Efficacy of Silexan (WS® 1265) in Patients with Restlessness and Sleep Disturbance. Annual Congress of the German Society for Psychiatry and Psychotherapy (DGPPN); November 24-27, 2010; Berlin, Germany 2010.
- Kasper S, et al: Int Clin Psychopharmacol 2010; 25(6): 277-287.
- Kasper S, et al: Int J Neuropsychpharmacol 2014; 17(6): 859-869.
- Lecrubier Y, et al: Am J Psychiatry 2002; 159(8): 1361-1366.
- Kasper S, et al: BMC Medicine 2006 June; 23: 4-14.
- Szegedi A, et al: BMJ 2005; 330(7490): 503-506.
- Kasper S, et al: Eur Neuropsychopharmacol 2008; 18(11): 803-813.
HAUSARZT PRAXIS 2015; 10(11): 24-27