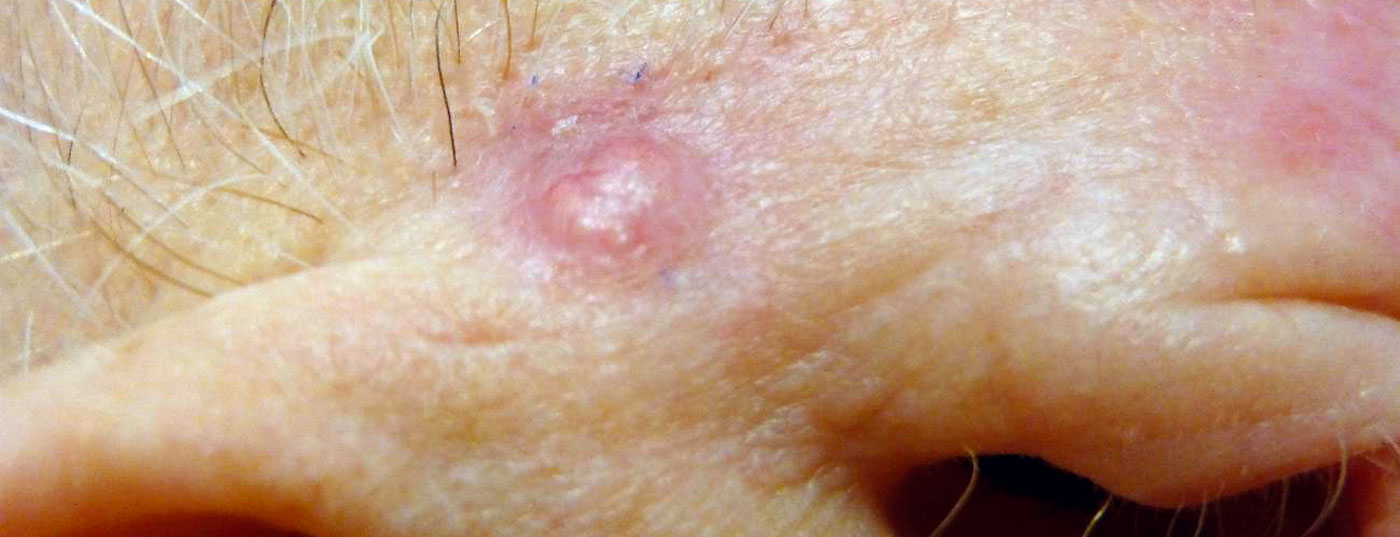
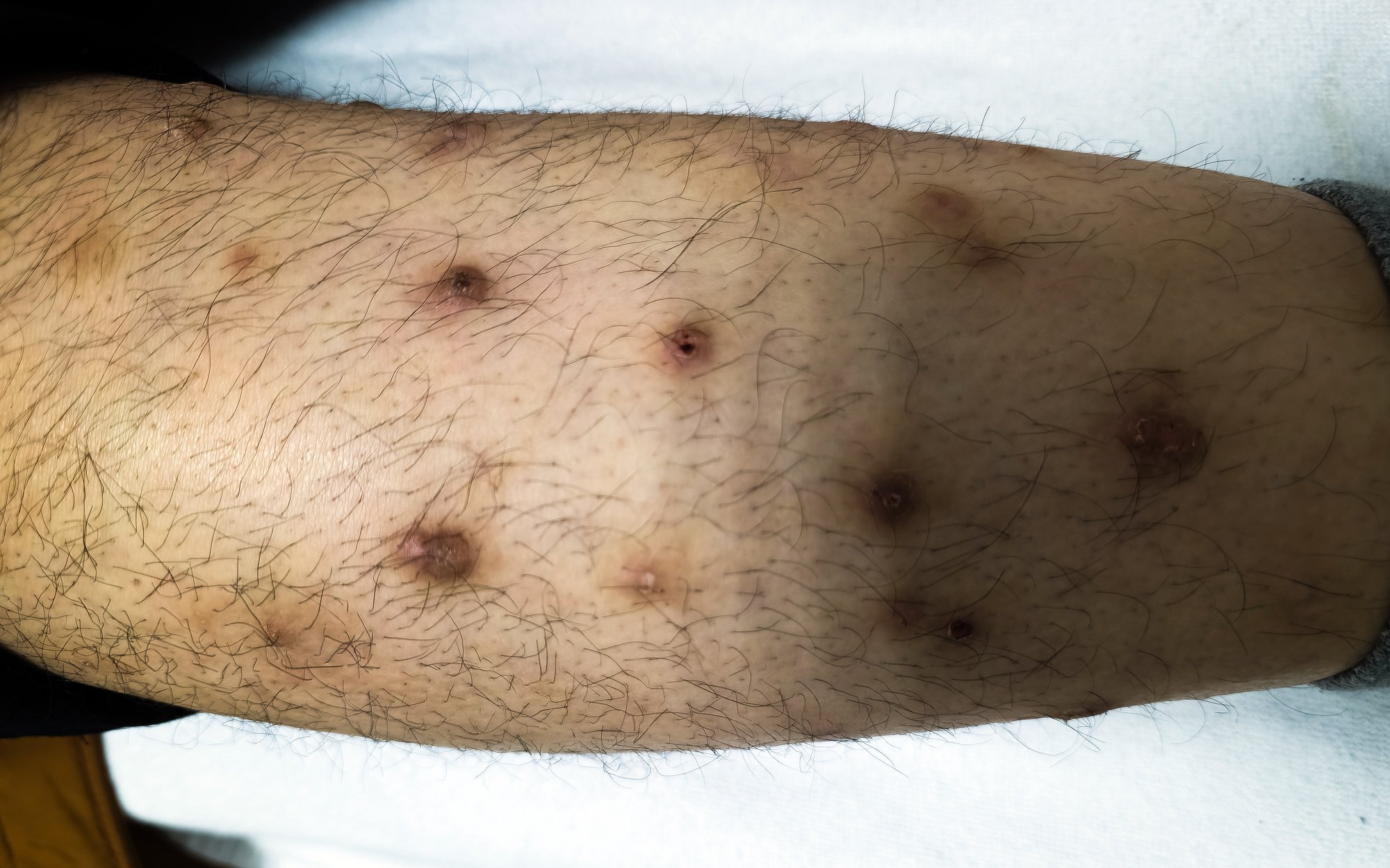
A review article published in the journal Cancer in 2024 discusses the current state of research on risk factors for the three most common types of skin cancer and highlights opportunities for individualized risk assessment. In addition to a compilation of the statistical parameters for melanoma, basal cell carcinoma and squamous cell carcinoma, validated risk scores are presented.
Skin cancer is the most common malignant disease worldwide in population groups with skin types I-III (Fitzpatrick classification). The incidence of melanoma and non-melanocytic skin cancer (NMSC) has increased in recent decades and will continue to rise in the future [1]. The main risk factor is cumulative exposure to ultraviolet radiation (UV) [2]. However, there is a need for further research to better understand the individual risk factors associated with skin cancer as a basis for improving early detection and treatment. Based on the current evidence, it is not clear whether whole-body skin cancer screening in asymptomatic individuals is effective at the population level in reducing morbidity and mortality, according to the authors [1,3]. They suggest that the individual risk of patients should be taken into account when making screening recommendations in order to make the diagnosis and treatment of skin cancer more efficient [4].
What is known from epidemiological research?
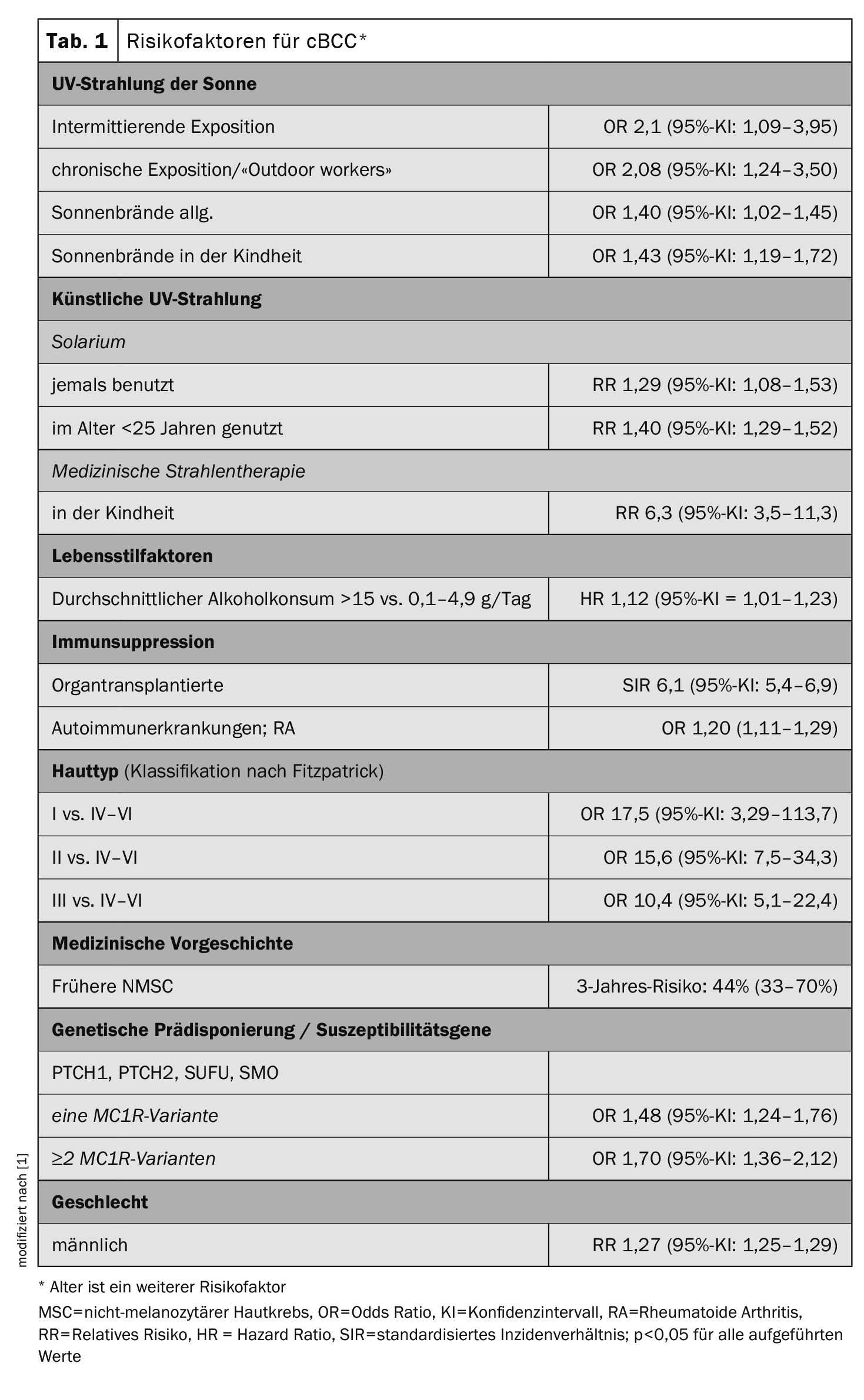
Individual risks for skin cancer are caused by environmental and behavioral factors as well as genetic predisposition. The specific risk factors for melanoma, basal cell carcinoma (cutaneous basal cell carcinoma, cBCC) and spinalioma (cutaneous squamous cell carcinoma, cSCC) vary. Wunderlich et al. In their overview article, the authors go into detail about the facts known from epidemiological research and summarize the most important findings for these three most common forms of skin cancer [1]. The full title of their review is “Risk Factors and Innovations in Risk Assessment for Melanoma, Basal Cell Carcinoma, and Squamous Cell Carcinoma” . The summary of epidemiological study-based data on odds ratio, hazard ratio, relative risk, and standardized incidence ratio for cBCC risk factors shown in Table 1 is also available for the other two skin cancer types in the original article [1].
Risk prediction models as a basis for early detection?
Risk prediction models aim to provide a comprehensive analysis that takes into account factors such as genetic predisposition, environmental influences and clinical characteristics to more accurately determine skin cancer risk. Various approaches use extensive data sets to construct models that are intended to facilitate early detection. Unfortunately, these models are often not sufficiently validated and do not take into account all known risk factors [5]. Some newer approaches attempt to overcome these problems.
Risk stratification based on “23andMe”: The authors of standardized incidence ratio “23andMe” developed a validated disease risk score based on a questionnaire and genetic data that could be used in screening programs. The risk score is based on 31 risk factors. This age-independent score predicts the occurrence of skin cancer (cBCC, cSCC, melanoma) in people aged 30 and over. Participants in the top percentile were not only diagnosed on average 10-14 years earlier than those with average scores, but also had more severe and recurrent forms of skin cancer. The calculated score and lifetime risk trajectories could be used in screening programs to identify asymptomatic individuals at high risk of skin cancer and predict when the highest risk of disease is likely to occur [6].
SUNTRAC tool: Organ transplant patients represent a special patient group. NMSC is the most common malignancy in solid organ transplant recipients and a major cause of morbidity and mortality [7,8]. According to the authors, there is therefore a need to develop risk scores that identify people who are particularly at risk. The Skin and UV Neoplasia Transplant Risk Assessment Calculator (SUNTRAC) tool was developed in the USA to facilitate the identification of solid organ transplant recipients with an increased risk of skin cancer by categorizing patients into risk groups. The risk factors (white ethnicity, skin cancer history, age ≥50 years, male gender, thoracic transplantation) were assigned weighted point values, resulting in a four-level system. The cumulative 5-year incidence for the development of skin cancer was 1.01%, 6.15%, 15.14% and 44.75% for the low, medium, high and very high categories respectively (SUNTRAC). The instrument was externally validated and tested for its applicability in European populations, with similarly good prognostic discrimination results. This model could help prioritize and ensure better screening and monitoring for these patients and define screening guidelines for organ transplant recipients [9,10].
In addition, the clinical benefit of these instruments in identifying people with an increased risk of skin cancer is currently being evaluated in general; results are still pending.
Non-coding RNAs (ncRNAs): ncRNAs are RNAs that are not transcribed into proteins. Only DNA transcription takes place, but the RNA is processed further. There is evidence that ncRNAs regulate important tumor pathways and play a role in almost all human tumors, including skin cancer. These functional RNA molecules, which have no protein-coding activity, act at transcriptional, post-transcriptional and epigenetic levels and are involved in cancer cell proliferation, angiogenesis, invasion and metastasis [11,12]. It has been shown that ncRNAs play a crucial role in the early diagnosis, prognosis and treatment of melanoma [13,14].
A recent meta-analysis showed a combined sensitivity of long ncRNAs in the diagnosis of melanoma of 72.4%, with a pooled specificity of 81.2% and an overall area under the curve (A UC) of 0.837. Using prognostic approaches, the HR for overall survival, progression-free survival and disease-free survival were 2.723 (95% CI: 2.259-3.283), 2.913 (95% CI: 2.050-4.138) and 2.760 (95% CI: 2.009-3.792) respectively [14]. These results suggest that ncRNAs could serve as innovative diagnostic and prognostic biomarkers that could improve the treatment of patients in the future, according to the authors.
Literature:
- Wunderlich K, et al.: Risk Factors and Innovations in Risk Assessment for Melanoma, Basal Cell Carcinoma, and Squamous Cell Carcinoma. Cancers (Basel). 2024 Feb 29; 16(5): 1016.
- Leiter U, Keim U, Garbe C: Epidemiology of Skin Cancer: Update 2019. Adv Exp Med Biol 2020; 1268: 123–139.
- Wolff T, Tai E, Miller T: Screening for skin cancer: An update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2009; 150: 194–198.
- Navarrete-Dechent C, Lallas A: Overdiagnosis of Melanoma: Is It a Real Problem? Dermatol Pract Concept 2023; 13:e2023246.
- Usher-Smith JA, et al.: Risk prediction models for melanoma: A systematic review. Cancer Epidemiol Biomark Prev 2014; 23: 1450–1463.
- Fontanillas P, et al.: Disease risk scores for skin cancers. Nat Commun 2021; 12: 160. doi: 10.1038/s41467-020-20246-5.
- Friman TK, et al.: Cancer risk and mortality after solid organ transplantation: A population-based 30-year cohort study in Finland. Int J Cancer 2022; 150: 1779–1791.
- Garrett GL, et al.: Trends of skin cancer mortality after transplantation in the United States: 1987 to 2013. JAAD 2016; 75: 106–112.
- Jambusaria-Pahlajani A, et al.: Predicting skin cancer in organ transplant recipients: Development of the SUNTRAC screening tool using data from a multicenter cohort study. Transpl Int 2019; 32: 1259–1267.
- Gómez-Tomás Á, et al.: External Validation of the Skin and UV Neoplasia Transplant Risk Assessment Calculator (SUNTRAC) in a Large European Solid Organ Transplant Recipient Cohort. JAMA Dermatol 2023; 159: 29–36.
- Kaushik SB, Kaushik N: Non-coding RNAs in skin cancers: An update. Non-coding RNA Res 2016; 1: 83–86.
- Durante G, et al.: Non-coding RNA dysregulation in skin cancers. Essays Biochem. 2021; 65: 641–655.
- Hasan MN, et al.: Hypoxia-related Y RNA fragments as a novel potential biomarker for distinguishing metastatic oral melanoma from non-metastatic oral melanoma in dogs. Vet Q 2024; 44: 1–8.
- Masrour M, et al.: Long non-coding RNA as a potential diagnostic and prognostic biomarker in melanoma: A systematic review and meta-analysis. J Cell Mol Med 2024; 28:e18109. doi: 10.1111/jcmm.18109.
DERMATOLOGIE PRAXIS 2024; 34(3): 22–23