Disorders of sleep as well as cognitive performance increase with age. Sleep and cognition are closely interrelated. Therefore, in terms of good everyday functionality, the treatment of sleep disorders in old age is of high importance.
With increasing age, there is a decrease in physical and mental performance. Changes in metabolic processes and the increased incidence of diseases are responsible for this. Both factors affect sleep as well as cognitive performance: Sleep disturbances increase with age, while mental fitness declines. Sleep – especially its individual parts – is closely related to this.
How sleep changes with age
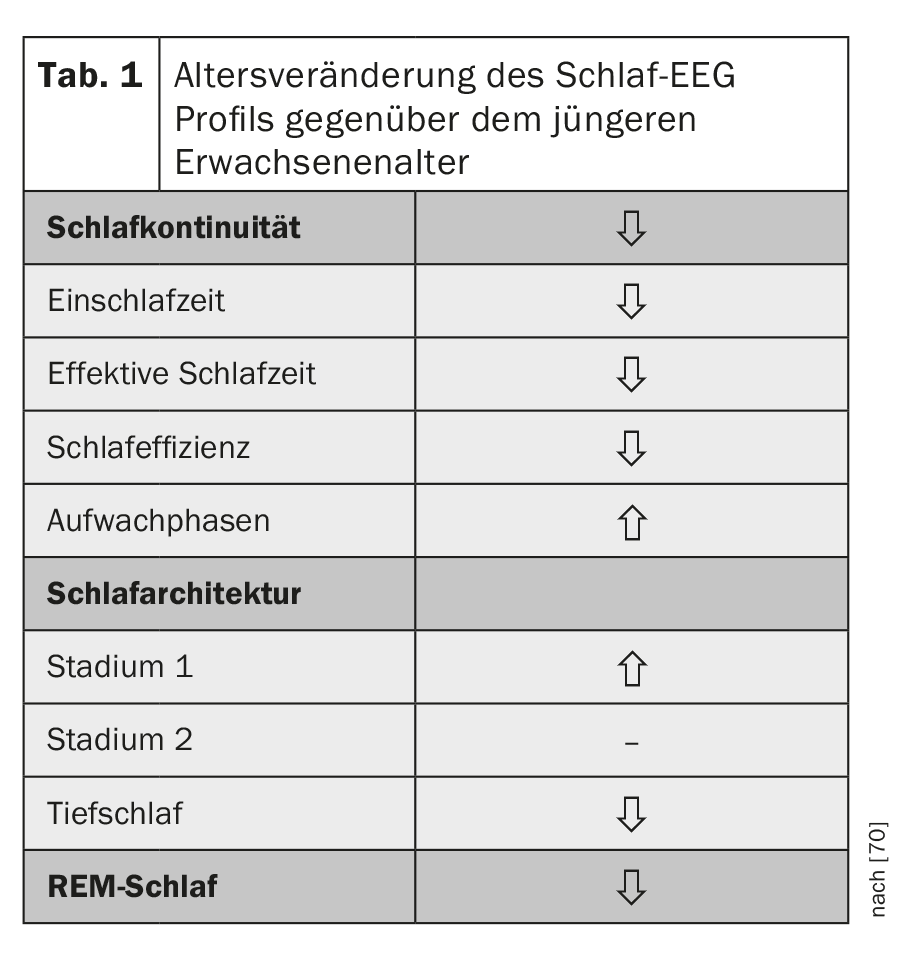
As we age, sleep becomes shorter and lighter. Objectification of these observable sleep disturbances by sleep EEG derivation shows that sleep continuity is characterized by lower sleep efficiency with longer time to fall asleep and more frequent nocturnal wakefulness. Sleep is less deep than in young adulthood, the light sleep stages of non-REM sleep (stages 1 and 2) occur more frequently, and deep sleep as well as REM sleep, although less pronounced than deep sleep, decrease with age [1,2] (Table 1).
Sleep-wake regulation and the regulation of non-REM and REM sleep are subject to neurochemical and neuroendocrine processes coupled to circadian and homeostatic processes. Cortisol is of great importance for the former, and growth hormone (GH) for the latter. Both hormones are centrally regulated by neuropeptides that include growth hormone-releasing hormone (GHRH), somatostatin, and CRH and vasopressin [3,4]. With age, the secretory activity of the GH-GHRH component decreases, making non-REM sleep less deep. These changes in both neuroendocrine axes are associated with lighter and shortened sleep with increased awakenings and less deep sleep with age (Fig. 1).
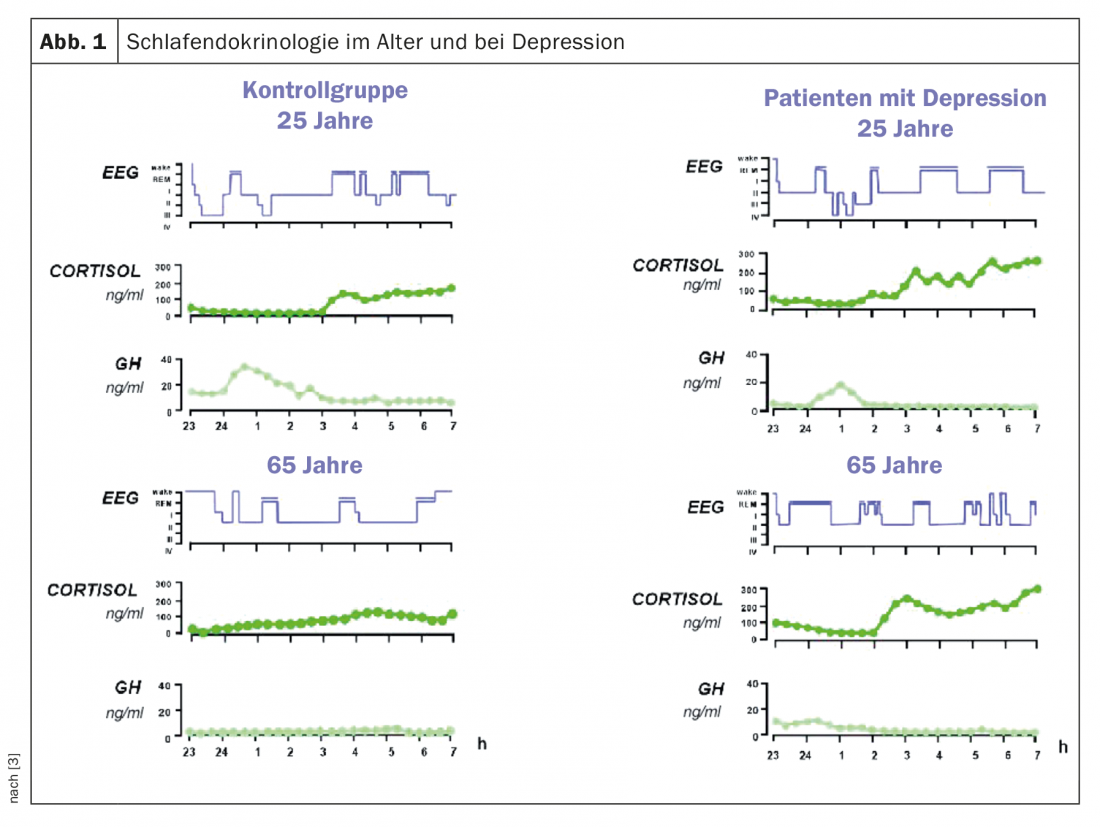
The circadian component of sleep regulation also changes with age. The circadian rhythms of core body temperature, melatonin, and cortisol are shifted forward by approximately one hour in the elderly (“phase advance”) with flatter amplitude, reduced by up to 30% [5]. This also leads to lighter and more restless sleep with early awakenings. A reduction of neuronal activity in the nucleus suprachiasmaticus, the endogenous circadian pacemaker, is considered to be the neuropathological basis of this circadian change [6].
As a result of these age-related changes in sleep-associated hormonal secretion of cortisol and growth hormone and altered circadian rhythms, there is an increased susceptibility to disruption of sleep by exogenous factors (stressors).
Cognitive “fitness” also declines
In addition to the pure memory function, cognitive performance also includes the functions of attention, linguistic skills, executive functions in the sense of planning and problem-solving thinking and acting, as well as visual-constructive or orienting skills and psychomotor speed [7].
The change in cognitive performance affects individual components of cognition in different ways as part of the normal aging process: the performance of working memory and episodic memory (knowledge of subjective experiences) declines. Less affected are semantic memory, autobiographical memory, recall of previously learned information, and emotional memory [8,9].
In contrast, access to semantic memory contents concerning factual knowledge or “knowledge about the world”, as well as the speed of access to them, do not seem to be impaired [10]. An exception is semantic information, which is relearned. Like episodic information, it is subject to an age effect [11].
The neuropathological substrate for the cognitive changes in healthy aging is thought to be a reduced capacity of the frontostriatal circuit, while changes in the medial temporal memory system are seen as the basis for the cognitive impairment in Alzheimer’s disease [8,9]. At the neurochemical and neuroendocrine level, especially the secretion of cortisol and changes in dopaminergic and cholinergic neurotransmission are closely related to the function of declarative memory (includes semantic, episodic, and autobiographical memory) in the elderly [9,12,13].
In contrast to declarative memory, procedural memory – the learning of complex automatic sequences of actions such as skiing – is largely independent of hippocampal structures [14] and is only slightly impaired with age, although acquisition is slower [11]. Neuropathologically, neostriatal and cerebellar brain regions are of particular relevance, showing age-related reductions in volume and metabolic glucose rate [15–18].
Performance in executive tasks, such as the trail-making test, shows a clear age effect, especially with respect to speed [19]. The prefrontal cortex in particular is considered to be the general neurobiological substrate for this. In addition, there is a general continuous reduction in the speed of cognitive processes with age, especially after the age of sixty [20].
How are sleep and memory related?
Disturbed sleep leads to deterioration of cognitive performance [21,22]. This can affect all cognitive dimensions, but preferentially episodic memory, working memory, and executive functions [23].
Previous studies in healthy subjects already showed that the neurophysiological activity of individual sleep stages is closely related to specific aspects of cognitive functions [24,25]. With few exceptions, a relationship between sleep and declarative memory performance in particular is found. This shows a correlation between the frequency of sleep spindles and deep sleep with memory consolidation [26,27]. Emotionally toned memory content seems to particularly benefit from undisturbed sleep, but seems to be associated with REM periods rather than deep sleep [28]. Here, interactions between prefrontal cortex and hippocampus, which can be interpreted as memory transfer from hippocampus to neocortex, play a major role [29].
The observation that sleep deprivation leads to suppression of long-term potentiation and neurogenesis in the hippocampus [30], supports this assumption. A short nap during the day also has positive effects on declarative memory consolidation [31,32].
For procedural memory, associations are found with sleep stage 2 [33,34] and REM sleep [35]. After sleep deprivation in the second half of the night, healthy subjects showed poorer procedural memory performance than under undisturbed sleep. In contrast, sleep deprivation in the first half of the night has no effect on procedural memory performance [26]. A positive effect has a short nap [36,37], provided that it includes REM sleep [38].
These results indicate an active role of sleep in memory formation-in the sense of promoting memory consolidation.
Mentally fit through deep sleep
In a large longitudinal study (more than 6000 individuals over 65 years of age without cognitive impairment), after three years of observation, cognitive impairment occurred primarily in those individuals who had reported sleep disturbance at baseline [39]. Especially executive functions seem to deteriorate during the aging process. They may be further exacerbated by (experimental) sleep deprivation or even a (chronically existing) sleep disorder [40].
Other sleep-associated impairments involve the functions of attention and vigilance. Individual studies provide evidence that sleep-dependent memory consolidation is impaired with age, but have been able to show that older individuals who have some deep sleep also have memory consolidation [41]. Therefore, the reduction in deep sleep is thought to be related to reduced cognitive performance, especially executive functions [40].
Sleep disturbance and reduced cognition: some clinical pictures.
In addition to the susceptibility to developing primary insomnia with increasing age, the incidence of insomnia as a consequence of other physical (e.g., pain syndromes, metabolic disorders, respiratory or cardiovascular diseases) and psychiatric disorders (e.g., depression, anxiety disorders) also increases. It should also be taken into account that drug treatment of these diseases can also have an impact on sleep (e.g., theophyline preparations for bronchial asthma) [42].
The development of insomnia in old age could be shown by the longitudinal study EPESE. After a three-year follow-up, 57% of the elderly reported at least one chronic sleep disorder, particularly a disorder of falling asleep or staying asleep [43]. Further data analysis showed a strong association between insomnia and the occurrence of depressed mood, breathing disorders, poor health, or physical disability. Similar results can be found for Switzerland [44]. In addition, there are specific primary sleep disorders such as restless leg syndrome (RLS) or sleep-disordered breathing [45,46], which can lead to further impairments in cognitive performance [47].
In addition to these primary or secondary disorders of sleep, psychiatric disorders, especially neuropsychiatric disorders, in which both disorders of cognition and disorders of sleep are part of the symptomatology, are also found in the elderly population. Primary among these are depression, the various dementias, and Parkinson’s disease, including REM sleep behavior disorder.
Depression in old age: Next to dementia, depression is the most common clinical picture in geriatric and neuropsychiatry. If mild depressive episodes are also included, prevalences of up to 25% are reported in various studies [48]. In depression, a characteristic change in the sleep profile is found, which is characterized by a prolonged time to fall asleep, a disturbance in sleeping through the night, and early waking. Sleep architecture shows a reduction in deep sleep and an increase and advance in REM sleep [49,50].
Cognitive disorders that have been described in patients with depression in old age are disorders of attention and psychomotor function, executive function, and verbal and visual learning and memory [51]. These disorders are associated with higher relapse rates and a more unstable course [52]. Chronic existing sleep disorders are also associated with up to a fourfold increased risk of depression [53].
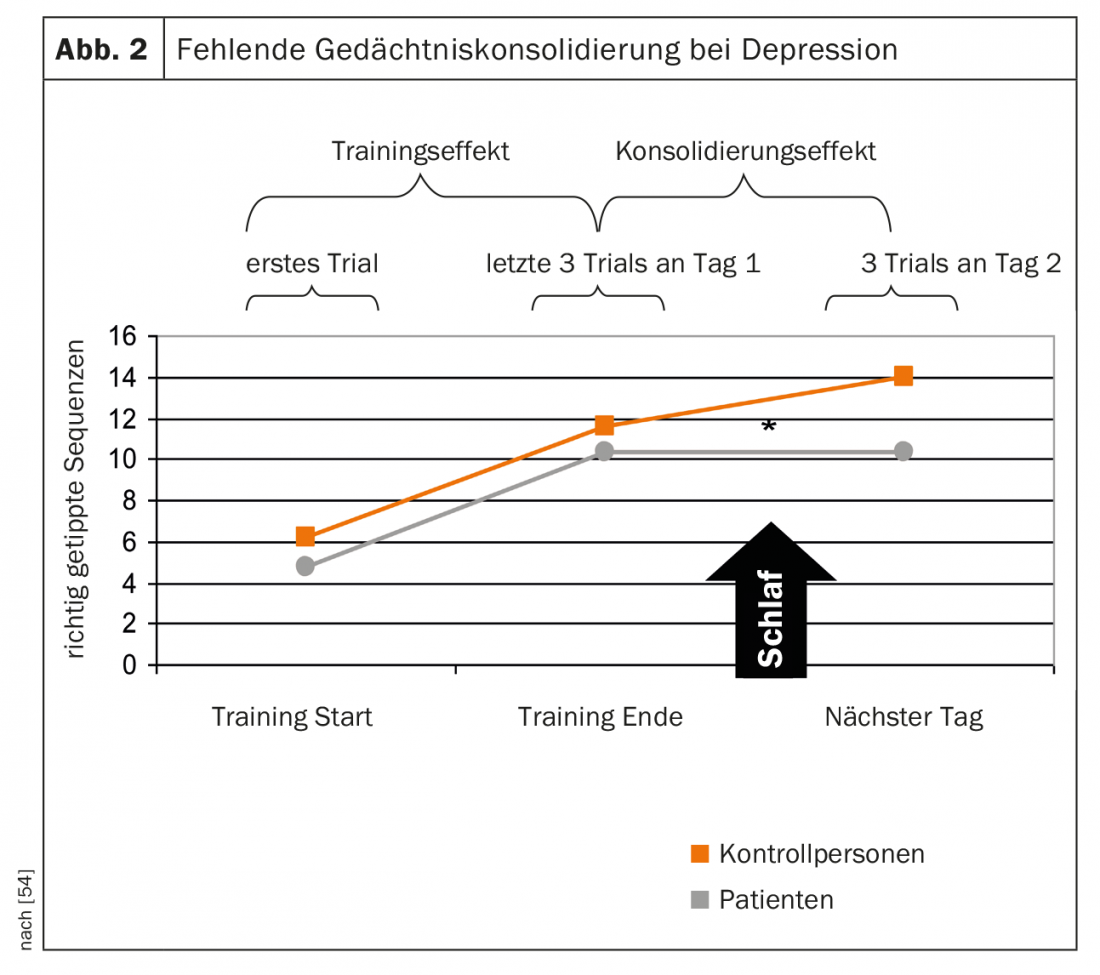
It is likely that impaired sleep continuity and reduced deep sleep negatively affect cognition, here predominantly sleep-associated memory consolidation, in patients with depression. This was also demonstrated in a study of sleep-associated memory consolidation in depressed patients in that, unlike healthy subjects, there was no overnight learning gain on a task learned before sleep [54] (Fig. 2).
Mild cognitive impairment (MCI) and dementia: Patients with dementia also frequently suffer from sleep disorders. The group of patients with Alzheimer’s dementia has been best studied. In addition to a disturbance in sleep continuity, other characteristic features found include a reduction in REM sleep throughout the night, lighter sleep, and in some of these patients, reduced deep sleep and fewer sleep spindles [55,56]. In studies of the relationship between sleep EEG parameters and cognition in this patient group, a strong association was found between poorer cognitive performance, especially in declarative memory, and reduced spindle activity [57] and reduced stage 2 [58].
Moreover, it has been shown that long-standing sleep disturbances lead not only to cognitive impairment but also to an increased incidence of manifest dementia [59,60]. A possible basis for this observation could be the recently found association between the occurrence of amyloid beta (Aβ as a neuropathological substrate of AD) with reduced sleep duration over years (<6 hours) in the elderly [61]. One possible mechanism discussed is that sleep disturbance prevents the brain from performing its clearance function for toxic metabolites such as Aβ and tau via the glymphatic system A at night [62].
Parkinson’s disease and REM sleep behavior disorder (RBD): RBD is characterized by a lack of inhibition of physiologically decreased muscle tone during REM sleep and an associated increase in muscle activity. As a result, motor movements occur during REM sleep. This results in the acting out of dream content to the point of behavior that is harmful to others or to oneself [63]. RBD, which is one of the parasomnias, can be seen as a symptom of Parkinson’s disease or Lewy body dementia. However, it can also occur symptomatically in the case of further brain damage of any kind (ischemia, tumors, MS), due to inflammatory processes or also under the administration of certain drugs (e.g. antidepressants). Furthermore, it has been described as an isolated clinical picture in terms of idiopathic RBD (iRBD) [64].
Additional sleep problems in RBD tend to occur in the advanced disease course and are characterized by significantly decreased total sleep time. In addition, cognitive deficits occur mainly at the level of declarative memory and visuoconstruction, as well as in executive functions [65]. In addition to the possible presence of RBD, sleep disturbances occurring in patients with PD depend on both the duration and the severity and progression of the disease [64]. In addition to a reduction in sleep efficiency or total sleep time, a disturbance in sleep architecture also occurs [66,67]. On the cognitive level, patients with PD regularly develop deficits in parts of executive functions, working memory, and declarative memory. In up to 50% of patients, dementia develops during the course of the disease [68,69].
Since sleep-sensitive domains of cognitive performance are impaired in both diseases, it can be assumed that there is a connection here.
Conclusions and outlook
The close relationship between sleep and cognitive performance suggests better cognitive performance with good sleep continuity and undisturbed sleep architecture with physiological and sufficient distribution of deep sleep, REM sleep, and other structures of sleep such as sleep spindles.
Disorders of sleep as well as cognitive performance increase overall with age. Sleep disturbance can be a relevant factor for the development of cognitive disorders up to dementia, depression and other somatic diseases (metabolic disorders, metabolic syndrome). For this reason, sleep disorders in the elderly should be detected quickly and treated consistently. This also applies to patients with the previously described diseases who have disturbances of sleep and cognition as symptoms.
Take-Home Messages
- The influence of sleep on cognitive performance is empirically proven. Not only the phase of falling asleep and sleeping through but also deep sleep and REM phases as well as microelements such as sleep spindles and K-complexes are closely related to specific cognitive functions.
- Various parameters of sleep change as part of the aging process. There is evidence that this interacts with an age-correlated decline in cognitive performance.
- Of particular relevance are those sleep disorders that play a role in diseases with impaired cognitive functions that occur more frequently in old age, such as dementia and old-age depression.
Literature:
- Ohayon MM, et al: Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep 2004; 27(7): 1255-1273.
- Vitiello MV: Sleep in normal aging. Sleep Medicine Clinics 2006; 1: 171-176.
- Steiger A: Sleep endocrinology. Neurologist 1995; 66: 15-27.
- Steiger A: Neurochemical regulation of sleep. J Psychiatr Res 2007; 41(7): 537-552.
- Pace-Schott EF, Spencer RM: Age-related changes in the cognitive function of sleep. Prog Brain Res 2011; 191: 75-89.
- Hofman MA, Swaab DF: Living by the clock: the circadian pacemaker in older people. Ageing Res Rev 2006 Feb; 5(1): 33-51. Epub 2005 Aug 25.
- Zimbardo PG: Psychology. Berlin, Heidelberg: Springer 1995.
- Buckner RL: Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron 2004; 44(1): 195-208.
- Hedden T, Gabrieli JD: Insights into the aging mind: a view from cognitive neuroscience. Nat Rev Neurosci 2004; 5(2): 87-96.
- Mayr U, Kliegl R: Complex semantic processing in old age: does it stay or does it go? Psychol Aging 2000; 15(1): 29-43.
- Prull MW, Gabrieli JDE, Bunge SA: Age-related changes in memory: a cognitive neuroscience perspective. In: Craik FIM, Salthouse SA (Eds.): The Handbook of Aging and Cognition 2000 (second edition); pp. 91-153. NJ: Lawrence Erlbaum Associates, Mahwah.
- Lupien SJ, et al: Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci 1998; 1(1): 69-73.
- Terry AV, Buccafusco JJ: The cholinergic hypothesis of age and Alzheimer’s disease-related cognitive deficits: recent challenges and their implications for novel drug treatment. J Pharmacol Exp Ther 2003; 306(3): 821-827.
- Squire LR, Zola SM: Structure and function of declarative and nondeclarative memory systems. Proc Natl Acad Sci USA 1996; 93(24): 13515-13522.
- Brickman AM, et al: Striatal size, glucose metabolic rate, and verbal learning in normal aging. Brain Res Cogn 2003; 17(1): 106-116.
- Doyon J, et al: Experience-dependent changes in cerebellar contributions to motor sequence learning. Proc Natl Acad Sci USA 2002; 99(2): 1017-1022.
- Laforce R, Doyon J: Differential role for the striatum and cerebellum in response to novel movements using a motor learning paradigm. Neuropsychologia 2002; 40: 512-517.
- Raz N, et al: Differential aging of the human striatum: longitudinal evidence. Am J Neuroradiol 2003; 24: 1849-1856.
- Mayr U, Kliegl R, Krampe RT: Sequential and coordinative processing dynamics in figural transformations across the life span. Cognition 1996; 59(1): 61-90.
- Salthouse TA: When does age-related cognitive decline begin? Neurobiol Aging 2009; 30(4): 507-514.
- Van Dongen HPA, et al: Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep 2004; 27(3): 423-433.
- Gadie A, et al.: How are agerelated differences in sleep quality associated with health outcomes? An epidemiological investigation in a UK cohort of 2406 adults. BMJ Open 2017; 7:e014920. doi: 10.1136/bmjopen-2016-014920.
- Fortier-Brochu E, et al: Insomnia and daytime cognitive performance: a meta-analysis. Sleep Med Rev 2012; 16(1): 83-94. doi: 10.1016/j.smrv.2011.03.008
- Born J, Plihal W: Memory formation during sleep: the importance of sleep stages and stress hormone release. Psychological Review 2000; 51198-51208.
- Stickgold R, James L, Hobson JA: Visual discrimination learning requires sleep after training. Nature Neuroscience 2000; 3(12): 1237-1238.
- Plihal W, Born J: Effects of early and late nocturnal sleep on declarative and procedural memory. Journal of Cognitive Neuroscience 1997; 9(4): 534-547.
- Gais S, et al: Sleep transforms the cerebral trace of declarative memories. Proc Natl Acad Sci USA 2007; 104(47): 18778-18783.
- Wagner U, Gais S, Born J: Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn Mem 2001; 8(2): 112-119.
- Mölle M, Born J: Hippocampus whispering in deep sleep to prefrontal cortex–for good memories? Neuron 2009; 61(4): 496-498.
- Guzman-Marin R, et al: Sleep deprivation suppresses neurogenesis in the adult hippocampus of rats. Eur J Neurosci 2005; 22(8): 2111-2116.
- Schabus M, et al: Sleep spindles and their significance for declarative memory consolidation. Sleep 2004; 27(8): 1479-1485.
- Lahl O, et al: An ultra short episode of sleep is sufficient to promote declarative memory performance. J Sleep Res 2008; 17(1): 3-10.
- Nader R, Smith C: A role for stage 2 sleep in memory processing. In: Maquet P, Smith C, Stickgold R (eds.): Sleep and Brain Plasticity; 2003. New York: Oxford Press.
- Manoach DS, et al: Reduced overnight consolidation of procedural learning in chronically medicated schizophrenia is related to specific sleep stages. J Psychiatr Res 2010; 44(2): 112-120.
- Marshall L, Born J: The contribution of sleep to hippocampus-dependent memory consolidation. Trends Cogn Sci 2007; 11(10): 442-450.
- Backhaus J, Junghanns K: Daytime naps improve procedural motor memory. Sleep Med 2006; 7(6): 508-512.
- Korman M, et al: Daytime sleep condenses the time course of motor memory consolidation. Nat Neurosci 2007; 10(9): 1206-1213.
- Tucker MA, et al: A daytime nap containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiol Learn Mem 2006; 86(2): 241-247.
- Cricco M, Simonsick EM, Foley DJ: The impact of insomnia on cognitive functioning in older adults. J Am Geriatr Soc 2001; 49(9): 1185-1189.
- Della Monica C, et al: Rapid Eye Movement Sleep, Sleep Continuity and Slow Wave Sleep as Predictors of Cognition, Mood, and Subjective Sleep Quality in Healthy Men and Women, Aged 20-84 Years. Front Psychiatry 2018; 9: 255.
- Backhaus J, et al: Midlife decline in declarative memory consolidation is correlated with a decline in slow wave sleep. Learn Mem 2007; 14(5): 336-341.
- Hemmeter UM: Management of sleep-wake regulatory disorders in diseases of aging. Swiss Archives of Neurology and Psychiatry 2011; 162(3): 108-118.
- Foley DJ, et al: Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep 1995; 18: 425-432.
- Delini-Stula A, Bischof R, Holsboer-Trachsler E: Sleep behavior in the Swiss population: Prevalence and the day-time consequences of insomnia. Somnology 2007; 11: 193-201.
- Ancoli-Israel S, et al: Periodic limb movements in sleep in community-dwelling elderly. Sleep 1991; 14: 496-500.
- Ohayon MM, Roth T: Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. J Psychosom Res 2002; 53: 547-554.
- Leng Y, McEvoy CT, Allen IE, Yaffe K: Association of Sleep-Disordered Breathing With Cognitive Function and Risk of Cognitive Impairment: A Systematic Review and Meta-analysis. JAMA Neurol 2017; 74(10): 1237-1245.
- Hatzinger M, et al: Recommendations for diagnosis and treatment of depression in the elderly. Praxis (Bern 1994) 2018; 107(3): 127-144.
- Riemann D, Berger M, Voderholzer U: Sleep and depression–results from psychobiological studies: an overview. Biol Psychol 2001; 57(1-3): 67-103.
- Benca RM, Peterson MJ: Insomnia and depression. Sleep Med 2008 Sep; 9 Suppl 1: S3-9.
- Reppermund S, Ising M, Lucae S, Zihl J: Cognitive impairment in unipolar depression is persistent and non-specific: further evidence for the final common pathway disorder hypothesis. Psychol Med 2009; 39(4): 603-614.
- Fossati P, Ergis AM, Allilaire JF: Executive functioning in unipolar depression: a review. Encephale 2002; 28(2): 97-107.
- Baglioni C, et al: Sleep and mental disorders: A meta-analysis of polysomnographic research. Psychol Bull 2016; 142(9): 969-990.
- Dresler M, et al: A double dissociation of memory impairments in major depression. J Psychiatr Res 2011; 45(12): 1593-1599.
- Bliwise DL: Sleep disorders in Alzheimer’s disease and other dementias. Clin Cornerstone 2004; 6(Suppl 1A): S16-28.
- Palmer K, et al: Sleep Disturbance in Mild Cognitive Impairment and Association With Cognitive Functioning. A Case-Control Study. Front Aging Neurosci 2018;10: 360.
- Rauchs G, et al: Is there a link between sleep changes and memory in Alzheimer’s disease? Neuroreport 2008; 19(11): 1159-1162.
- Kundermann B, et al: Comparison of polysomnographic variables and their relationship to cognitive impairment in patients with Alzheimer’s disease and frontotemporal dementia. J Psychiatr Res 2011; 45(12): 1585-1592.
- Ju YE, Lucey BP, Holtzman DM: Sleep and Alzheimer disease pathology-a bidirectional relationship. Nat Rev Neurol 2014; 10: 115-119. doi: 10.1038/nrneurol.2013.269
- Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA: Sleep fragmentation and the risk of incident Alzheimer’s disease and cognitive decline in older persons. Sleep 2013; 36: 1027-1032. doi: 10.5665/sleep.2802
- Busche MA, Kekuš M, Förstl H: How sleep and Alzheimer’s disease are related. Neurologist 2017; 88(3): 215-221.
- Xie L, et al: Sleep drives metabolite clearance fromthe adultbrain. Science 2013; 342: 373-377.
- Boeve BF: REM sleep behavior disorder: Updated review of the core features, the REM sleep behavior disorder-neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann N Y Acad Sci 2010; 1184: 15-54.
- Oertel WH, et al: REM sleep behavior disorder as a prodromal stage of α-synucleinopathies: symptoms, epidemiology, pathophysiology, diagnosis and therapy. Neurologist 2014; 85(1): 19-25.
- Hemmeter UM: REM sleep disturbance in dementia and Parkinson’s disease. Leading Opinions Neurology and Psychiatry 2018; 4: 15-17.
- De Cock VC, Vidailhet M, Arnulf I: Sleep disturbances in patients with parkinsonism. Nat Clin Pract Neurol 2008; 4(5): 254-266.
- Placidi F, et al.: Sleep-wake cycle and effects of cabergoline monotherapy in de novo Parkinson’s disease patients. An ambulatory polysomnographic study. J Neurol 2008; 255(7): 1032-1037.
- Foltynie T, et al: The cognitive ability of an incident cohort of Parkinson’s patients in the UK. The CamPaIGN study. Brain 2004; 127(Pt 3): 550-560.
- Balas M, Giladi N, Gurevich T: Cognition in multiple system atrophy: neuropsychological profile and interaction with mood. J Neural Transm 2010; 117(3): 369-375.
- Hemmeter UM: Sleep and cognition in old age. Psychiatry & Neurology 2012; 4: 10-14.
InFo NEUROLOGY & PSYCHIATRY 2019; 17(1): 10-15.











