Topical treatment of plaque psoriasis has a high priority in the everyday clinical practice. In mild symptoms, topical topical preparations are the mainstay of therapy; in moderate to severe cases, they are used as an adjunctive measure. A Swiss expert group of dermatologists has developed practice recommendations with the aim of optimizing topical therapy for psoriasis patients.
The expert panel consensus recommendations include guidance on general aspects of treatment as well as specific recommendations on basic care, induction and maintenance therapy for plaque psoriasis [1]. These were published in the Journal Dermatology 2021 (“Topical Treatment of Psoriasis Vulgaris: The Swiss Treatment Pathway”) [1]. This article is a summary of them.
A short- and long-term treatment strategy is one of the success factors, along with the choice of suitable active ingredients and galenics. Clarifying expectations of treatment and establishing shared goals for therapy between physician and patient increase adherence.
Promotion of compliance and monitoring of the course of the disease
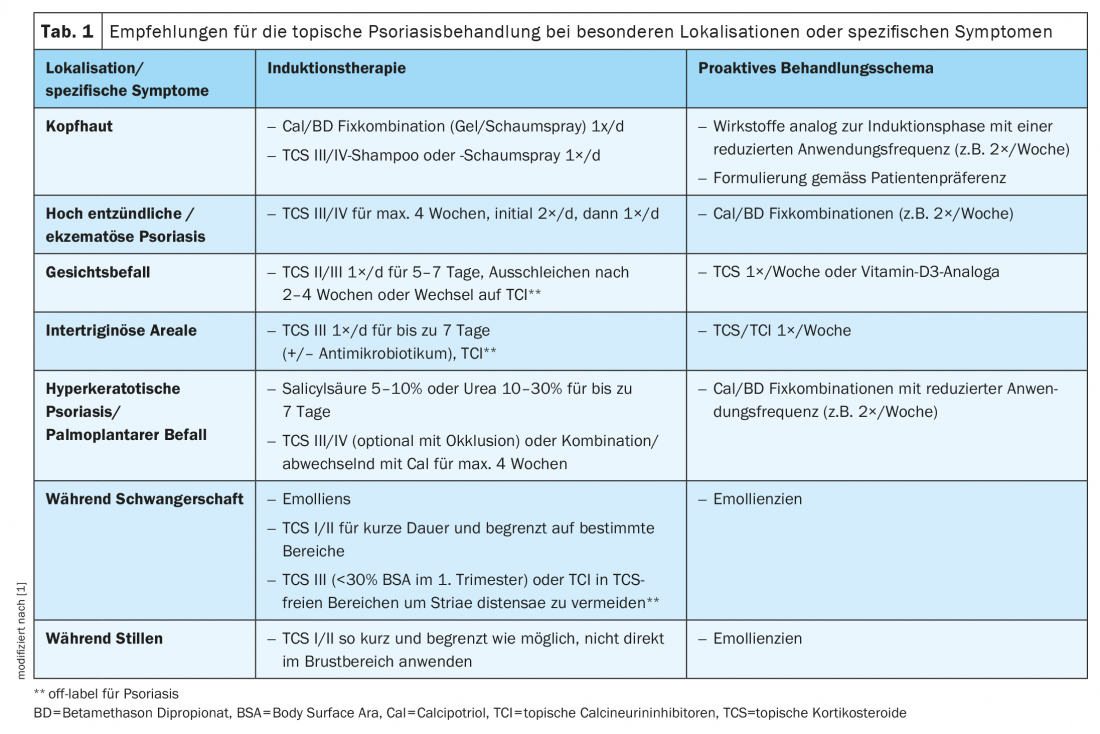
Personal preferences and previous experience of the patient must be taken into account when choosing the treatment. In addition, special therapy adjustments are required with regard to specific localizations and symptomatology (Tab. 1). The treatment regimen should be kept as simple as possible. Written instructions on dosage and frequency of therapy may favorably influence compliance [2,3].
Daily basic therapy with emollients is important in all stages of the disease for restoring epidermal barrier function, as well as for improving elasticity and maintaining the microbial balance of the skin [4]. Preparations containing urea 5-10%, salicylic acid, ceramides, niacinamide or thermal water have been shown to be beneficial for the basic therapy of lesional or non-lesional psoriatic skin [5]. Basic care measures must also be continued in the induction and maintenance phase.
During the induction and maintenance phases, progress monitoring should be performed every 12 weeks. If treatment goals are not achieved after 4-8 weeks, one of the following options may be considered in consultation with the patient:
- Continuation of therapy in view of possible therapy response at a later point in time
- Switch to a secondline or thirdline option that better matches the patient’s symptoms and treatment preferences
- Referral of the patient to a treatment center specializing in psoriasis. If topical therapy does not lead to sufficient symptom control within a useful time, the use of UV therapy or systemic treatment (according to the current Swiss S1 guideline) [6] should be considered.
Induction therapy
For the induction phase in mild to moderate psoriasis, topical calcipotriol (Cal) 50 μg/g and betamethasone dipropionate 0.5 mg/g once daily for a period of 2-8 weeks is most commonly used. With regard to the dosage form, patient preferences must be taken into account [3].
After 4-8 weeks, the treatment goal of lesion-free/almost lesion-free skin should be achieved. Subsequently, one can gradually reduce the frequency of application (e.g., every other day for another 2 weeks) and proceed to maintenance therapy (e.g., twice weekly therapy application).
Alternatively, monotherapy with class III/IV topical corticosteroids (TCS) or topical vitamin D3 analogues may be considered as second-line treatment [7,8]. In certain situations, TCS may also be considered as first-line treatment. It is recommended that TCS be continued as monotherapy (once/twice daily application) for no longer than 4 weeks.
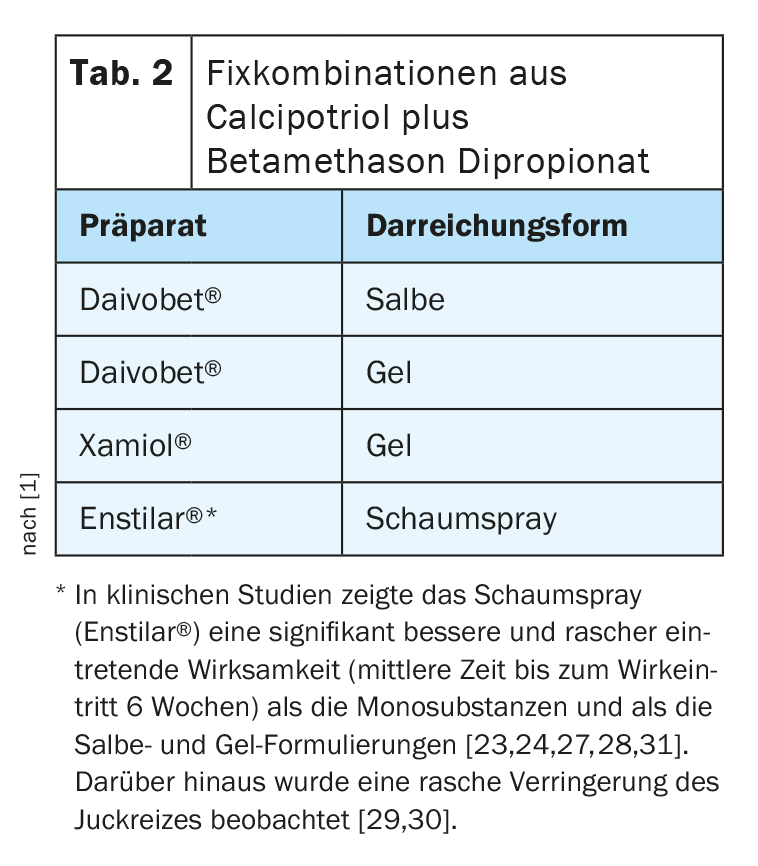
Fixed combinations with calcipotriol and betamethasone dipropionate: The benefit of using fixed combinations of calcipotriol (Cal) plus betamethasone dipropionate is based on additive effects regarding reduction of keratinocyte hyperproliferation and inflammatory processes as well as synergistic effects regarding tolerability (lower risk of skin atrophy and less burning) compared to the administration of the monosubstances [9]. Currently, three formulations of fixed combinations (Tab. 2) are approved, which do not differ in terms of concentration of ingredients, but only the foam spray unfolds a supersaturation of the completely dissolved active ingredients on the skin and an associated improved skin penetration and bioavailability [10–12]. The foam spray (Enstilar®) [13] is also rated by patients as particularly user-friendly in application.
Topical corticosteroids (TCS): The use of class I to IV TCS (Table 3) as monotherapy or combined with vitamin D3 analogues has long been part of the standard therapy for mild psoriaisis. TCS are characterized by immunosuppressive and cell proliferation inhibitory effects. When choosing the formulation (ointment, cream, solution, lotion, foam, shampoo), an individual choice should be made taking into account the patient’s preferences. In general, TCS is mostly used once a day, preferably in the evening. Once the therapeutic goal has been reached, the corticosteroids can be phased out.
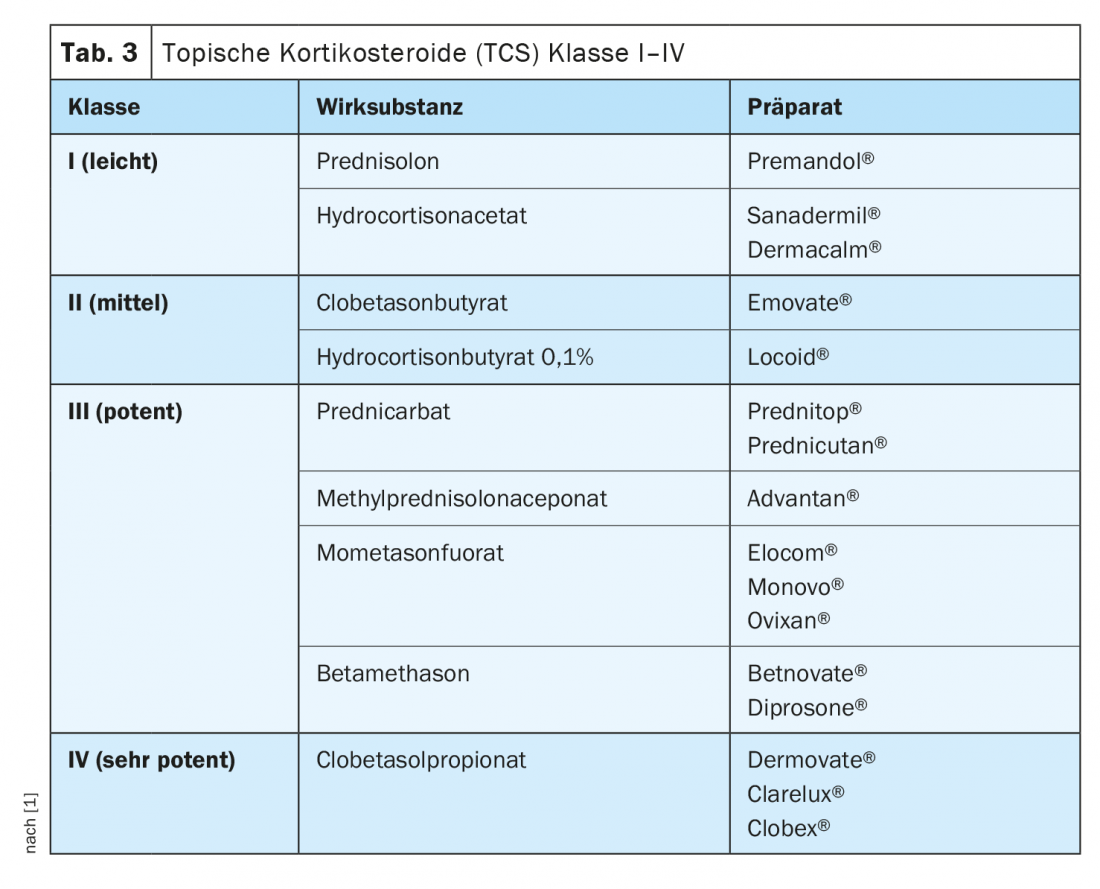
The occurrence of undesirable side effects varies depending on the active substance class of the corticosteroid as well as the localization and the duration of application. The most frequently reported is burning of the skin. Long-term use of Class III/ IV TCS may result in, among other things, telangiectasia. If the frequency of application is increased and/or under occlusion, systemic effects such as adrenal suppression are also possible. No serious adverse events have been reported with the use of TCS in the setting of guideline-directed induction therapy.
Vitamin D3 analogs: vitamin D3 analogs and derivatives include tacalcitol (Curatoderm®) and calcitriol (Silkis®) [13]. These exert their effects via specific receptors in target cells of the skin, whereby the effects are selective and vary depending on the differentiation state of the keratinocytes [14]. In rapidly growing, non-differentiated keratinocytes, vitamin D3 analogues inhibit further growth, whereas in slowly growing keratinocytes there is a proliferation-promoting effect. Moreover, in addition to affecting keratinocytes, vitamin D3 analogs inhibit the development of PBMCs (“Peripheral Blood Mononuclear Cells, such as lymphocytes and monocytes”) in the peripheral blood and inhibit various cytokines [14].
Depending on the preparation, the application is carried out once or twice a day. When used according to guidelines, a body surface area of up to 10% is considered an indication area. Therefore, the restriction of the use of vitamin D3 analogues to a maximum of 15-35% of the total body surface area according to the expert information is not of practical relevance [1]. Possible adverse effects of vitamin D3 analogues include local irritation (pruritus, burning, erythema). No clinically relevant disturbances of calcium metabolism are to be expected if the specified maximum amounts are observed.
Maintenance therapy
After successful induction therapy, it is possible to transition to the maintenance regimen. The same active substances should be used as in the induction phase, but at a lower frequency (e.g. 2×/week). Standardized and simplified treatment regimens result in better clinical outcomes than demand-driven therapies [3,15].
The best evidence for successful and effective long-term topical treatment is for the use of fixed combinations of calcipotriol (Cal) plus betamethasone dipropionate. In a recent phase III study, twice-daily proactive use of the foam spray of calcipotriol plus betamethasone dipropionate reduced the risk of first relapse by 43% (95% CI 0.47-0.57), and the difference from the placebo group was found to be highly significant (p<0.001) [34].
Regarding long-term use of TCS in fixed combinations, current data do not indicate a large risk of skin atrophy in psoriasis patients, in contrast to atopic eczema. According to experimental studies, the combined use of vitamin D3 analogues and TCS attenuates the risk of atrophy [16]. However, regarding long-term use, there is only a small evidence base of studies with validated and objective measurement parameters and biomarkers of atrophy. Long-term TCS monotherapy is not recommended.
In addition to the fixed combination of Cal plus betamethasone dipropionate, other treatment regimens including TCS class II/III as well as other vitamin D3 analogs may be considered as second- or third-line options for maintenance therapy. Their use is possible on a daily basis or limited to individual weekdays.
Special localizations and specific symptoms
Scalp involvement: scalp involvement is present in up to 79% of psoriasis patients [17–19]. The treatment of capillitium is often particularly challenging and strongly subject to patients’ personal preferences. The most important factor regarding patient adherence is the acceptability of the formulation and the perceived effectiveness.
In a 2016 Cochrane Review, Cal/ betamethasone dipropionate in fixed combination and TCS monotherapy were found to be more effective and safer for the treatment of scalp psoriasis than vitamin D3 monotherapy [19]. Available as gel and foam spray, the fixed combinations of Cal plus betamethasone dipropionate are well tolerated and completely water- and alcohol-free. The correct application procedure on the capillitium should be discussed with the patient before starting treatment. The use of alcoholic solutions should be avoided, as often accompanied by burning and additional drying of the scalp.
TCS Foam [20] or Shampoo [21] and Cal/ Betamethasone Dipropionate Gel [32,33] have been specifically developed for the treatment of psoriasis in the capillitum area. More recently, Cal/ betamethasone dipropionate foam spray has demonstrated its efficacy in the treatment of scalp psoriasis on several occasions [23–25]. The corresponding preparation approved in Switzerland is Enstilar®. The foam spray formulation has good patient acceptance and proved to be an effective treatment option for induction therapy (2-4 weeks). In the maintenance phase, the application frequency can be reduced to twice weekly and, depending on patient preference, a switch to the Cal/ betamethasone dipropionate fixed combination in gel form (Daivobet® Gel, Xamiol®) [13] can be considered.
Infestation of the facial skin: Lesions of the facial skin can be initially treated with a class II to III TCS once daily for 5-7 days. If there is a response to therapy within 2-4 weeks, TCS should be discontinued. The use of TCS (Class III) once a week is generally considered safe. Alternatively, topical calcineurin inhibitors (TCI, off-label) can be switched to avoid potential side effects of TCS such as skin atrophy. TCIs such as tacrolimus ointment and pimecrolimus cream affect the activation of T cells, keratinocytes, and mast cells. None of these preparations is approved for psoriasis, but several small studies showed their efficacy in psoriasis of the face and intertriginous areas [22,26]. Proactive therapy is recommended for the maintenance phase, as in atopic eczema. The use of topical vitamin D3 analogues may also be considered.
Palmoplantar psoriasis: Psoriatic lesions in palmoplantar localizations are characterized by hyperkeratotic, pustular or mixed manifestations and are associated with significant impairment of quality of life. The majority of patients with palmoplantar psoriasis are female and former or current smokers. In contrast to pustular forms of palmoplantar psoriasis, where topical therapy is often not effective, topical treatment may be a useful option for plaque-type palmoplantar psoriasis. This usually involves keratolytic treatment to reduce hyperkeratosis, followed by the use of potent to very potent class III/IV TCS. Initial occlusive therapy allows accelerated onset of action. After a treatment period of approximately 2-4 weeks, the use of a fixed combination of Cal plus betamethasone dipropionate may be considered.
Other particular localizations and special manifestations of psoriasis include infestation of intertriginous areas, highly inflammatory manifestation form, hyperkeratotic psoriasis, and pregnancy and breastfeeding. The corresponding therapy recommendations are summarized in Table 1.
Literature:
- Maul J-T, et al. Topical Treatment of Psoriasis Vulgaris: The Swiss Treatment Pathway. Dermatology 2021; 237: 166-178.
- Reich K, et al: Br J Dermatol 2017; 177(1): 197-205.
- Augustin M, et al: J Dtsch Dermatol Ges 2014; 12(8): 667-682.
- Luger T, et al: Eur J Dermatol 2014; 24(2): 194-200.
- Thaçi D, et al: JDDG 2015; 13(5): 415-418.
- Kolios AG, et al: Swiss S1 Guidelines on the Systemic Treatment of Psoriasis Vulgaris. Dermatology 2016; 232: 385-406.
- Samarasekera EJ, et al: Br J Dermatol 2013; 168(5): 954-967.
- Mason A, et al: JAAD 2013; 69(5): 799-807.
- Segaert S, Ropke M: J Drugs Dermatol 2013; 12(8): e129-137.
- Puig L, Carretero G: Actas Dermosifiliogr 2019; 110(2): 115-123.
- Basse LH, et al: J Invest Dermatol 2014; 134: 33.
- Lind M, et al: Dermatol Ther (Heidelb) 2016; 6(3): 413-425.
- Drug Information, www.swissmedicinfo.ch (last accessed Jun. 18, 2021).
- Wilsmann-Theis D, et al. Topical therapy of psoriasis, 1st edition – Bremen: UNI-MED, 2016.
- Piaserico S, et al: G Ital Dermatol Venereol 2018; 153(5): 692-697.
- Norsgaard H, et al: Poster presented at 21st EADV, 2012: No. PRA12-0845.
- van de Kerkhof PC, et al: Dermatology 1998; 197(1): 31-36.
- van de Kerkhof PC, et al: Dermatology 1998; 197(4): 326-334.
- Schlager JG, et al: Cochrane Database Syst Rev. 2016 Feb;2: CD009687.
- Payne J, et al: J Drugs Dermatol 2019; 18(8): 756-770.
- Reygagne P, et al: J Dermatolog Treat 2005; 16(1): 31-36.
- Jacobi A, et al: Dermatology 2008; 216(2): 133-136.
- Paul C, et al: JEADV 2017; 31(1): 119-126.
- Lebwohl M, et al: J Clin Aesthet Dermatol 2016; 9(2): 34-41.
- Anderko M, et al: Clin Cosmet Investig Dermatol 2019; 12: 699-705.
- Remitz A, et al: Br J Dermatol 1999; 141(1): 103-107.
- Koo J, et al: J Dermatolog Treat 2016; 27(2): 120-127.
- Leonardi C, et al: J Drugs Dermatol 2015; 14(12): 1468-1477.
- Jalili A, et al: JEADV 2019; 33(4):709-717.
- Pink AE, et al: JEADV 2019; 33(6): 1116-1123.
- Menter A, et al: Skinmed 2017; 15(2): 119-124.
- Jemec GB, et al: J Am Acad Dermatol 2008; 59(3): 455-463.
- van de Kerkhof PC, et al: Br J Dermatol 2009; 160(1): 170-176.
- Lebwohl M, et al: Long-term proactive management of psoriasis vulgaris with fixed-dose combination of calcipotriene 0.005% and betamethasone dipropionate 0.064% foam: results of a phase III randomized controlled trial; AAD 2020, Chicago, Poster # 18223.
DERMATOLOGY PRACTICE 2021, 31(4): 39-41