At the SHG Congress in Geneva, a satellite symposium discussed the current study situation in the field of non-valvular atrial fibrillation. How can stroke be effectively prevented and what is the benefit-risk profile of each agent? In particular, the focus was on practical reality. Apparently, there is still a need to catch up with regard to the implementation of study findings in everyday clinical practice – this despite the fact that the evidence leaves hardly any questions unanswered.
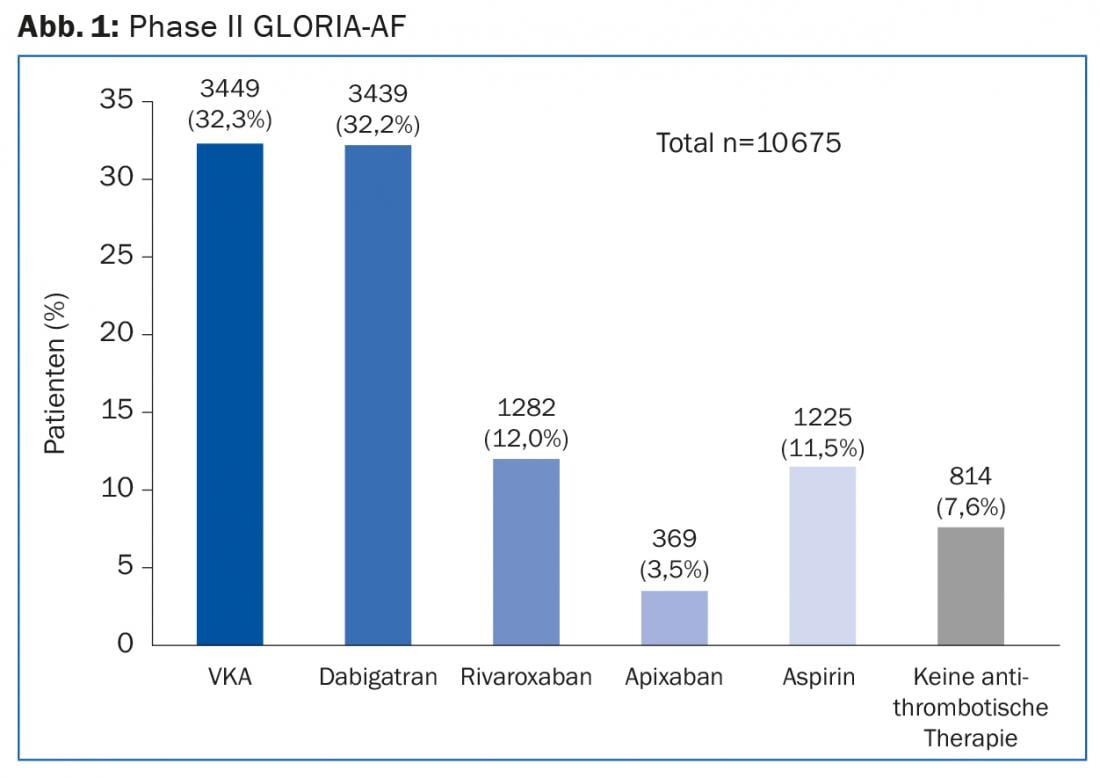
Discussion sometimes centered on which patient characteristics influence the choice of antithrombotic therapy. In addition, we again reviewed the collected safety and efficacy outcomes of each agent and discussed regional differences in their prescribing. The first topic was the large international registry study GLORIA-AF. This is a program that was launched in May 2011 and includes patients with newly diagnosed non-valvular atrial fibrillation (AF). The goal is to collect data from clinical practice – this will provide approximately 56,000 patients from nearly 50 countries (and over 2200 centers). All are at risk for stroke (CHA2DS2-VASc score ≥1). The cross-sectional data [1] presented at the 2014 ESC Congress in Barcelona covered a recruitment period from November 2011 to February 2014 and were based on 10,675 patient cases (85.5% of whom had a CHA2DS2-VASc score ≥2). They originate from the second phase of the GLORIA AF program. This started shortly after the approval of dabigatran, the first NOAK for stroke prevention in VHF.
Data from 39 countries show that although an increasing shift toward NOAKs is evident in clinical practice (Fig. 1), the process is slow and the rate of undertreated patients remains high. In addition, for newly diagnosed VCF, vitamin K antagonists (VKA) continue to be used most frequently, and aspirin still accounts for a significant proportion.
Acting against the evidence?
This is surprising, because the evidence speaks for itself: the NOAKs can be considered standard therapy for VHF. A review of the studies (RE-LY [2–4], ROCKET-AF [5], ARISTOTLE [6], ENGAGE-AF [7]) shows that NOAKs are superior to VKAs in the benefit-risk profile. To illustrate, according to a recent review of the RE-LY trial [4], dabigatran at the dose of 2× 150 mg/d showed a relative risk of stroke or systemic embolism of 0.65 (95%CI 0.52-0.81; p<0.001), a risk reduction of 35% compared with warfarin. For ischemic or nonspecific strokes, the value was 0.76 (95%CI 0.59-0.97; p=0.03).
According to GLORIA-AF, implementation of the findings into practice is going best in North America and Europe, where NOAKs are slowly overtaking VKAs. In Asia in particular (but also in North America in some cases), many patients – although at high risk of stroke – remain untreated or are treated exclusively with aspirin.
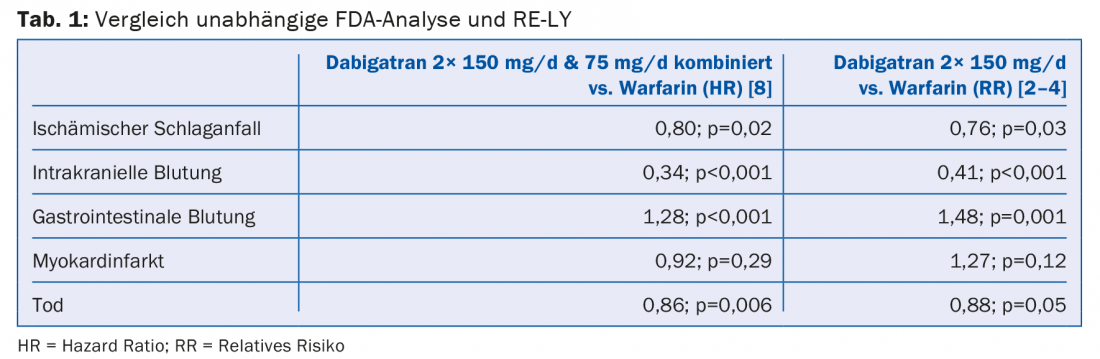
This approach contradicts the current state of studies. Concerns that the effects of NOAKs present differently in the randomized trial setting than in clinical reality have recently been refuted again by an independent FDA analysis [8]. It confirmed the good benefit-risk profile of dabigatran in the everyday practice setting with astonishing consistency: the data collected from 134 414 elderly patients were largely consistent with those from RE-LY (Table 1) .
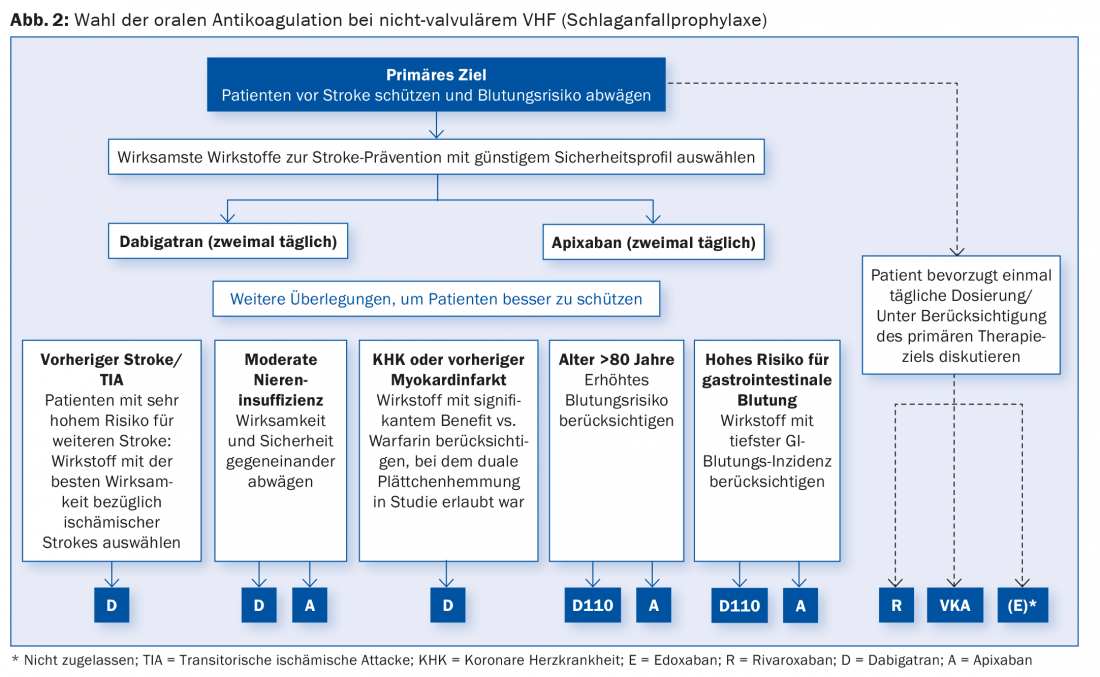
It is therefore important to now increasingly translate the evidence into clinical action. Using patient characteristics, Prof. Hans-Christoph Diener, Essen, MD, showed how this could be done. The algorithm is shown in Figure 2 .
Source: “Sheltering patients from stroke: optimizing protection,” Satellite Symposium at SHG Congress, January 29-30, 2015, Geneva.
Literature:
- Huisman MV, et al: Global registry on long-term oral antithrombotic treatment in patients with atrial fibrillation: baseline characteristics of the first 10,000 patients in GLORIA-AF Phase II. Session “Registry Hot Line: Atrial fibrillation and myocardial infarction”. ESC Congress 2014.
- Connolly SJ, et al: Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009 Sep 17; 361(12): 1139-1151.
- Connolly SJ, et al: Newly identified events in the RE-LY trial. N Engl J Med 2010 Nov 4; 363(19): 1875-1876.
- Connolly SJ, Wallentin L, Yusuf S: Additional events in the RE-LY trial. N Engl J Med 2014 Oct 9; 371(15): 1464-1465.
- Patel MR, et al: Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011 Sep 8; 365(10): 883-891.
- Granger CB, et al: Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011 Sep 15; 365(11): 981-992.
- Giugliano RP, et al: Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013 Nov 28; 369(22): 2093-2104.
- Graham DJ, et al: Cardiovascular, bleeding, and mortality risks in elderly medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation 2015 Jan 13; 131(2): 157-164.
HAUSARZT PRAXIS 2015; 10(4): 33-35