The main manifestation of symptomatic arterio-venous malformation (AVM) is intracranial hemorrhage, typically subarachnoid or intracerebral as a result of rupture in localized destructive vessel wall remodeling. Other typical symptoms exist either as irritable symptoms and often present as headache and epilepsy, or due to reduced circulation of adjacent brain areas resulting in non-hemorrhagic focal neurologic deficits. To attenuate the risk of the main risk of hemorrhage, the therapeutic goal is to exclude the AVM, which can be done endovascularly, surgically, radiosurgically, or by a combination of two or all three of these methods, depending on the circumstances. In the presence of nonhemorrhagic symptomatology such as headache, epilepsy, or focal neurologic deficits, partial occlusion may be considered for symptom improvement. In the presence of hemorrhagic symptoms, occlusion of a lesion (aneurysm) suspected as the source of bleeding may partially protect against further bleeding of the AVM at the same site, but complete occlusion of the AVM is preferable if possible with acceptable risk.
Arterio-venous malformations (AVM) are circumscribed vascular diseases, often of origin at birth, which may manifest during life with the consequences of multiple short-circuit connections between the arterial and venous systems. As with all neurovascular diseases, there is the possibility of interference with the adjacent normal cerebral circulation or directly with the brain.
AVM of the CNS are comparable in frequency and type to the equally rare AVM of other tissues, which are now usually treated only for their symptoms. The key difference lies in the nature of the clinical manifestation, which in AVM of the brain include hemorrhage and neurologic irritant symptoms (epilepsy, headache) or neurologic dysfunction – all conditions that severely limit quality of life. These risks, which accompany both the observational and the active approach, make the decision regarding active treatment or adopting a wait-and-see attitude in the case of an incidental finding of AVM not easy and often controversial.
Formation of a nidus
It is likely that AVM are already foetally created and subsequently become visible and proportionally larger as they grow. Today, MRI, which is increasingly performed foetal or in early childhood, rarely, and even then only incidentally, reveals specific forms of AVM. Therefore, it may be assumed that most AVM of the brain, although, as assumed, foetally well laid out, only become visible in the course of life and, depending on the localization, only manifest themselves secondarily.
Arterio-venous malformations include multiple, pathologically created short circuits (AV fistulas) between arteries and veins, i.e., without intervening blood pressure- and blood flow velocity-reducing capillary network. The resulting AV pressure gradient leads to an accelerated circulation, a so-called “AV shunt”. The consecutive suction leads to the genesis of numerous new vessels corresponding to the need and often collaterally involves neighboring arterial and venous vessel territories – a rich arterio-venous vascular network, a nidus, is formed. Depending on the structure and location of the AVM nidus and its relationship to the arteries and veins of the adjacent parts of the brain, it may be very compact and circumscribed, but it may also be diffusely configured, and in this case more difficult to distinguish from normal brain tissue.
Stenoses and ectasias
The increased circulation may lead to premature aging and wear of the affected vascular segment, resulting in the development of stenosis and vascular ectasia. Both types of wall changes are now associated with increased bleeding risk, arterial and venous ectasia as a site of weakened vessel walls and obstructive stenosis as an indicator of prestenotic potentially elevated pressure-circumstances that are best assessed during catheter angiographic examination. Even today, cross-sectional imaging methods largely miss this information, which is crucial for a decision to take active action.
Detect destructive processes in time
As in other neurovascular diseases, including aneurysm, atherothrombotic processes circumscribed adjacent to the vessel wall are now thought to be responsible for adjacent inflammatory changes that weaken the vessel wall [1, 2]. The difficulty is to detect these biological, destructive processes of wall remodeling (“destructive remodeling”) in time and correctly because of their subtlety in order to be able to prevent potential bleeding.
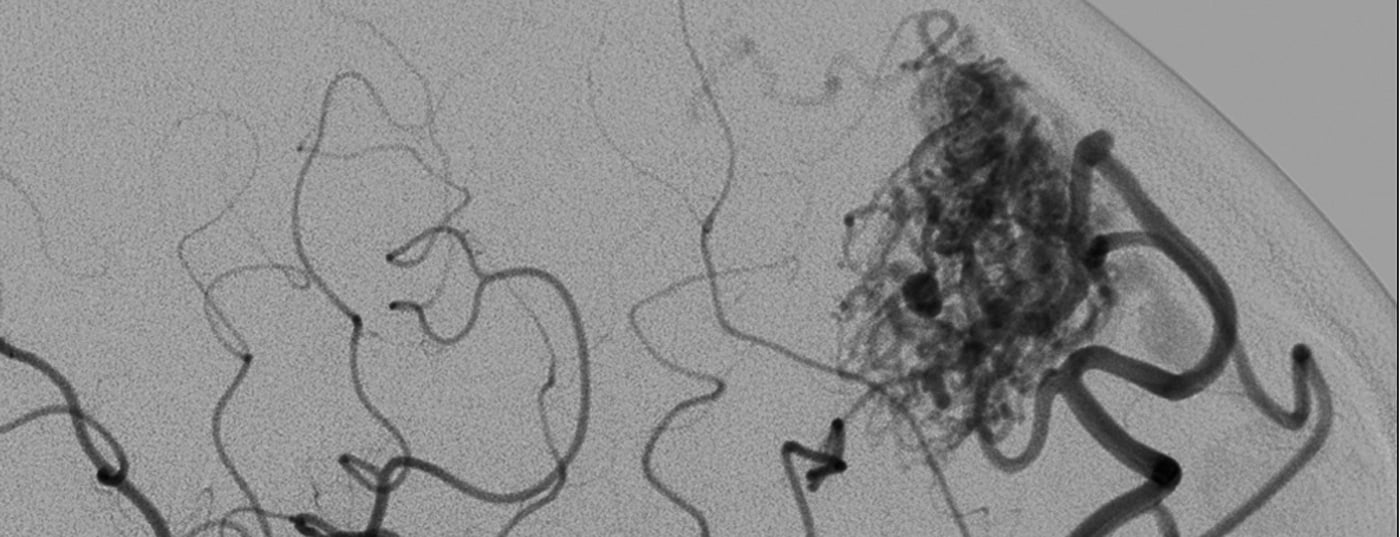
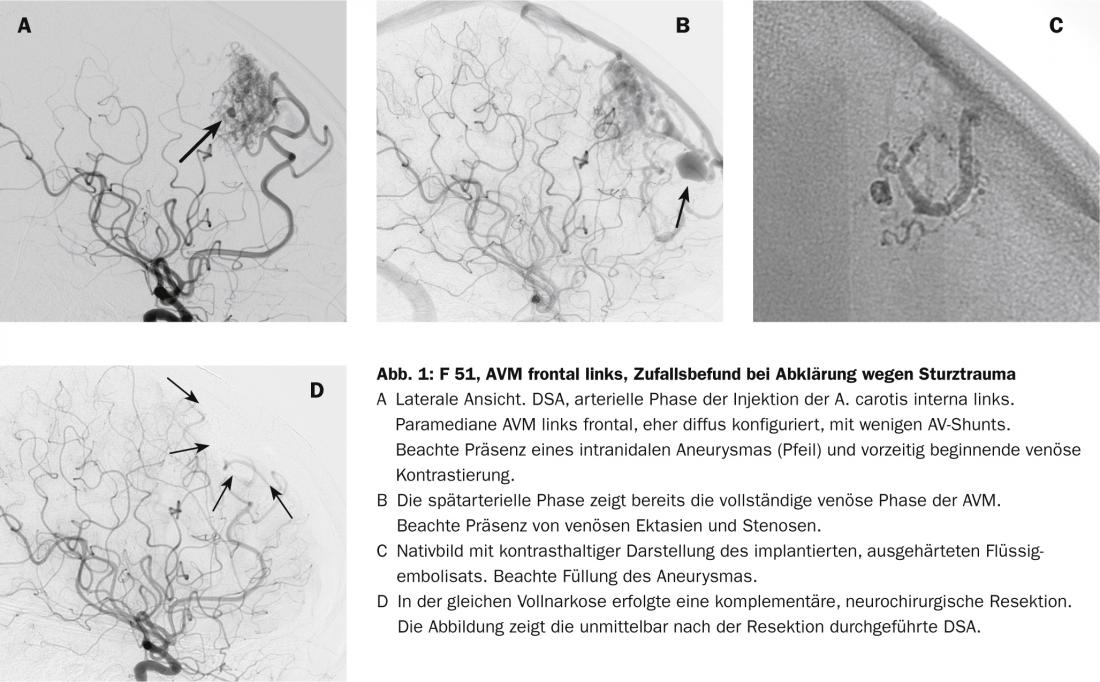
Because AVM are statistically rare, pathomorphology is highly variable, and biological behavior is additionally variable among individuals, reliable prediction of bleeding risk remains difficult [3–6]. In addition to size and location, vascular morphologic criteria that influence prognosis include arterial aneurysm formation, venous ectasia, and especially stenoses associated in venous outflow (Fig. 1). Although biological processes are suspected to be responsible for the bleeding tendency, there are no other reliable biological markers to date that can be used to reliably assess the status and potential course of vascular malformation [2].
The incidental finding
The current quality of medical imaging using magnetic resonance imaging (MRI) and computed tomography (CT) often allows identification of an AVM without specifically looking for it; the typical situation of an accidentally found, asymptomatic AVM arises.
The international multicenter RCT ARUBA (“A Randomized Trial of Unruptured Brain AVMs”; http://avm.ucsf.edu/patient_info/aruba_trial), initiated in 2007, compares the observational stance with active treatment and argues against an active approach to incidental findings according to currently known data. A detailed evaluation of the data obtained is expected shortly.
Although intracranial arteriovenous malformations are very rare, one must assume that the true incidence is somewhat underestimated with a currently estimated prevalence of 15 per 100000. The annual incidence (symptomatic presentation) of AV malformations is 1-2/100000 per year. For AVM that have not manifested by hemorrhage, differences in the various published series are relatively small, so that one can assume a risk of hemorrhage of between 2 and 4% per year. Once an AVM has bled, the risk for further bleeding increases.
Familial associations of AV malformations are seen clinically almost exclusively in association with Osler-Weber-Rendue syndrome (hereditary hemorrhagic telangiectasia, HHT) and otherwise occur sporadically. Thus, in the sporadic occurrence of AVM, there is usually no need to evaluate family members.
In any case, AV malformation should be clarified in detail by means of catheter angiography in order to detect and evaluate factors that pose an increased risk to the patient. The following parameters are recognized indicators of increased risk of bleeding for reasons stated above and from publications:
- Small AV malformation (nidus size <3 cm)
- Deep location (peri- or intraventricular, or in the basal ganglia).
- Deep venous drainage and venous ectasia associated with stenosis and associated aneurysms juxta- or intranidally.
If bleeding has already occurred, the risk of rebleeding is significantly increased, especially in the first six months [7].
The initial non-hemorrhagic symptoms
Epilepsy: Epileptic seizures are the initial symptom in over one third of patients. In most patients, seizures are focal or focal complex, with cortical AVM more commonly associated with seizures. Many patients can be controlled relatively well with medication.
Headache: Chronic headache is present in about 30% of patients with AVM. Endovascular obliteration of any meningeal arterial supply involvement usually results in a marked reduction in such headaches, suggesting meningeal irritability. Mechanisms such as irritation of the cerebral arteries leading to headache are discussed; the nervi vasorum of the proximal cerebral artery segments could possibly allow pain signals to be perceived.
Focal neurologic deficits: Focal neurologic deficits are also present in nearly one-third of patients with AVM. These can be progressive, stable or fluctuating. The mechanism of these focal deficits varies. Sometimes a postictal condition may hide such focal deficits, but more often there may be a circumscribed disturbance of cerebral circulation (“steal” phenomenon). Thus, sometimes a regional venous hypertension is probably behind it or also an irritative, pulsatile space-occupying effect of a vascular ectasia.
Therapy options
In principle, AV malformations are treatable by neurosurgery, neurointervention, or radiotherapy. The goal of all three treatments, either individually or in combination of two or three therapies, should be to completely close the AVM. The sometimes existing assumption that a reduction of AVM size also leads to a reduction of bleeding risk is not correct. On the contrary, a change in hemodynamics in an AVM (virtually inevitable consequence of a size reduction without complete occlusion) may well also lead to an increase in the risk of bleeding and should by no means lull one into a false sense of security.
In principle, the decision for or against a specific therapy of an AV angioma should be made on an interdisciplinary basis. In our opinion, neurosurgical therapy and interventional therapy alone or in combination play the greater role (Fig. 1). In deep-seated AVM or brainstem AVM supplied by multiple, perforating arteries, radiosurgery is much lower risk. Because of the latency between therapy and closure of the AVM after radiotherapy, surgical extirpation and endovascular therapy alone or in combination should be favored to achieve a rapid preventive effect.
Endovascular treatment
Endovascular superselective catheter access to an AVM, usually performed under anesthesia, is now a routine procedure and, as long as vessel dimensions permit, virtually any vessel branch can be approached. Superselective vessel lumen imaging allows fine analysis of vessel and nidus morphology while opening the option of endovascular closure. Polymerizing liquid adhesives or precipitating, low- or non-adhesive substances are usually used for this purpose. Under fluoroscopic, biplane visual control, these substances are injected gradually while avoiding occlusion of normal adjacent vessels.
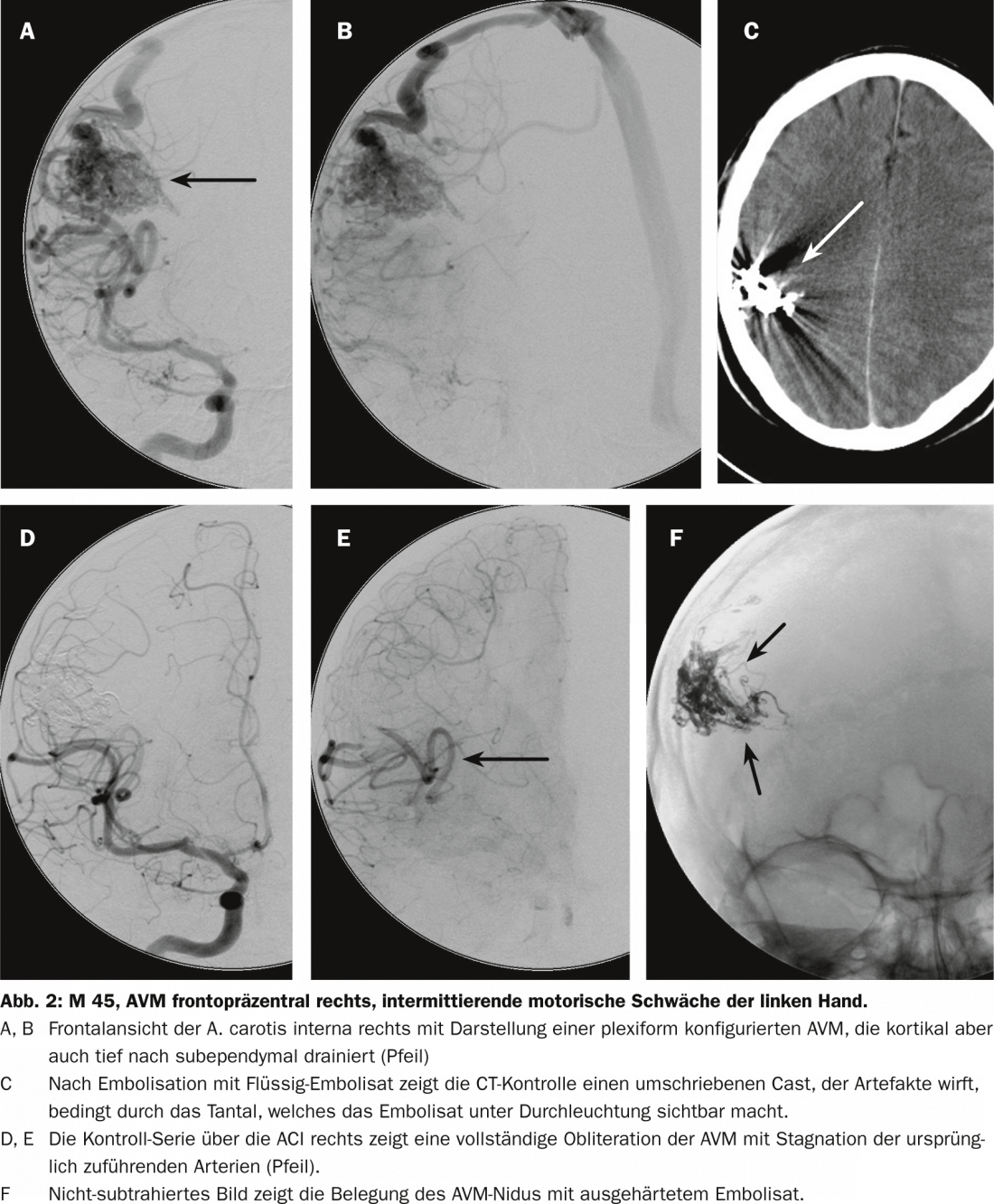
The risk lies in obliteration of normal vessels and bleeding due to injection and endovascular manipulation. These are risks that are taken only if they are considered proportional to the assessed spontaneous bleeding risk. We usually use a precipitating substance. Once the intranidal catheter position has been reached, it is slowly injected into the nidus. It is often possible to occlude large portions of the AVM or even the entire AVM via a single catheter position. However, depending on the compartmentation, it may of course be necessary to close such an AVM over two or three positions. The rate of complete occlusions has increased significantly since the use of such substances (Fig. 2) [8].
Neurosurgical treatment
Superficially located and small AV malformations can be operated on and completely removed with very low mortality and morbidity, whereas deeply located, large AV malformations draining through the brain parenchyma can be associated with dramatically higher surgical morbidity and mortality, up to more than 50%. A large meta-analysis in 2000 with over 2400 patients showed that the average mortality after neurosurgical extirpation was 3.3% and the average morbidity was 8.6% (1.5-18.7%) [9].
The neurosurgical treatment risk must be assessed individually using the Spetzler and Martin classification. Graduation of Spetzler-Martin AVM is based on size, venous drainage, and eloquence of surrounding brain tissue, based on catheter angiography, CT, or MR. Graduation of the lesion is determined by summing the individual points. The higher the score, the higher the neurosurgical operative risk for morbidity or mortality.
This classification does not provide a prognosis regarding the risks of any associated endovascular therapy.
Stereotactic radiosurgery
In the case of stereotactic radiotherapy performed in a single session as so-called radiosurgery, the gradual onset of radiation, up to max. The obliterative success of the treatment, which can be observed approximately five years after therapy, depends crucially on the size of the AVM and the diameter of the vessels supplying the AVM. For AVM with a nidus size less than 15 mm, the complete obliteration rate is 77%; if the nidus is between 15 and 25 mm, the obliteration rate is 62%, reducing to 44% if the nidus is larger than 25 mm. With additional dural inflows present, the probability of complete obliteration is only half as high. Delayed onset treatment complications of radiotherapy, predominantly due to radiation necrosis in adjacent brain tissue, are 5-7%, plus a persistent bleeding risk of 3-4% per year as long as closure of the AVM has not occurred.
Multidisciplinary collaboration
Consultation and treatment of brain AVM is best done in a multidisciplinary manner by a medical team consisting of interventional neuroradiologists, neurosurgeons, and radiotherapists associated with critical evaluation by neurologists and, when appropriate, neuropsychologists and psychiatrists. Posttherapeutic care is provided in close collaboration with neurointensivists and, if necessary, with a network of rehabilitation and social reintegration.
Preventive exclusion of hemorrhage by treatment of asymptomatic AVM is now questioned because the risks of invasive therapy are currently considered higher than those of spontaneous progression. Long-term care of patients and family members is thus necessary, especially when a decision is made to proceed non-actively, and a continuous doctor-patient relationship is beneficial.
Literature:
- Chen Y, et al: Evidence of inflammatory cell involvement in brain arteriovenous malformations. Neurosurgery 2008; 62(6): 1340-1349 (discussion 1349-1350).
- Kim H, et al: Genetic considerations relevant to intracranial hemorrhage and brain arteriovenous malformations. Acta Neurochir Suppl 2008; 105: 199-206.
- Laakso A, Hernesniemi J: Arteriovenous malformations: Epidemiology and clinical presentation. Neurosurg Clin N Am 2012; 23(1): 1-6.
- Laakso A, et al: Risk of hemorrhage in patients with untreated spetzler-martin grade IV and V arteriovenous malformations: A long-term follow-up study in 63 patients. Neurosurgery 2011; 68(2): 372-377; (discussion 378).
- Hernesniemi JA, et al: Natural history of brain arteriovenous malformations: A long-term follow-up study of risk of hemorrhage in 238 patients. Neurosurgery 2008; 63(5): 823-829 (discussion 829-831).
- Al-Shahi R, Warlow C: A systematic review of the frequency and prognosis of arteriovenous malformations of the brain in adults. Brain 2001; 124: 1900-1926.
- Stapf C, et al: Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology 2006; 66(9): 1350-1355.
- Panagiotopoulos V, et al: Embolization of intracranial arteriovenous malformations with ethylene-vinyl alcohol copolymer (onyx). AJNR Am J Neuroradiol 2009; 30(1): 99-106.
- Castel JP, Kantor G: Postoperative morbidity and mortality after microsurgical exclusion of cerebral arteriovenous malformations. Current data and analysis of recent literature. Neurosurgery 2001; 47(2-3): 369-383.