From December 7-10, 2021, the 44th San Antonio Breast Cancer Symposium took place in a physical-virtual hybrid event. In a series of exciting lectures, the latest clinical and preclinical findings on the treatment of breast carcinoma were presented and lively discussions were held. We have summarized the most important studies on locally advanced and metastatic breast cancer from the four General Sessions for you.
With the breakthrough superiority of trastuzumab-deruxtecan (T-DXd) over trastuzumab-emtansine in second-line HER2-positive tumors and the introduction of sacituzumab-govitecan in triple-negative breast cancer (TNBC) – just to name two examples – a lot has already happened in advanced/metastatic breast cancer in the last year. More research news was presented at the San Antonio Breast Cancer Symposium (SABCS) in December 2021.
Some of these could shape clinical practice in the near future – both in HER2-positive, hormone receptor (HR)-positive/HER2-negative and triple-negative breast cancer. In addition to new agents and indications, the spotlight was also on liquid biopsy for monitoring and adjusting therapies.
HER2-positive breast cancer: a clear target
The therapeutic target in HER2-positive tumors seems to be established. Thus, studies on HER2-targeted therapies were predominantly presented at the SABCS (Tab. 1) . As at previous congresses, there was one substance in particular that attracted enthusiasm: the antibody-drug conjugate trastuzumab-deruxtecan (T-DXd). This is now approved in Switzerland as monotherapy for those patients whose disease has progressed after at least two treatment regimens directed against HER2, including trastuzumab and trastuzumab emtansine (T-DM1) [1]. An admission that could change in the near future – and should, according to the results of the DESTINY-Breast03 study. This is because the phase III study, which has already been presented at the ESMO Congress 2021, showed a clear superiority of T-DXd in terms of progression-free survival (PFS) in a direct comparison with trastuzumab-emtansine in the second line of treatment after trastuzumab/taxane. While the 1-year PFS rate was 75.8% in the T-DXd group, it was 34.1% with trastuzumab emtansine therapy (hazard ratio HR 0.28, 95% confidence interval 0.22-0.37) [2]. New findings from a subgroup analysis were presented at the 44th SABCS. In summary, the advantage of T-DXd in terms of PFS and response rate was confirmed across all subgroups. It was clearly and impressively detectable regardless of hormone receptor status, prior pertuzumab administration, presence of visceral disease or clinically stable brain metastases, and number of prior therapies [3]. Thus, T-DXd also appears to be effective in brain metastases – an important signal considering that such develop in up to half of patients. A mixed preclinical/clinical study also presented at SABCS specifically analyzed the efficacy of T-DXd in active brain metastases – and came to the same conclusion. The efficacy of the antibody-drug conjugate could be confirmed in cell lines and first 16 patients with active brain metastases [4].
As another alternative therapy targeting HER2, a Chinese research team presented the new agent pyrotinib [5]. This is a tyrosine kinase inhibitor (TKI) targeting EGFR, HER2 and HER4. This was convincing in a previous study in combination with capecitabine versus lapatinib + capecitabine in the second line of therapy after trastuzumab/taxane [6]. On this basis, pyrotinib has already been approved in China. In an update, positive PFS as well as OS data from a phase III trial were presented at SABCS (Table 1) [5].
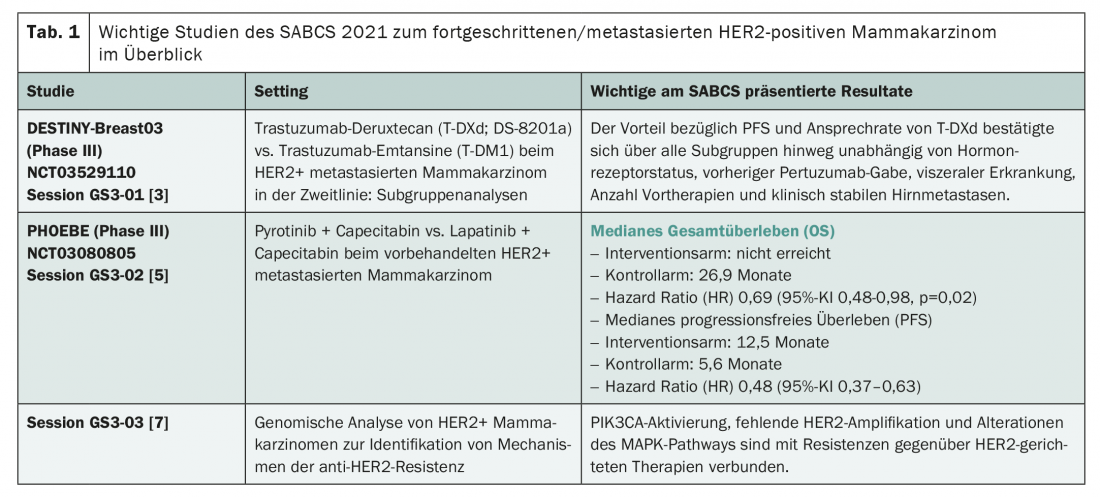
In addition to agents directed against HER2, the symposium also focused on research into anti-HER2 resistance mechanisms. These are becoming increasingly important as the use of appropriate therapies increases and can not only contribute to a broader understanding of treatment failure, but also provide proposed solutions – as demonstrated by a study presented at SABCS [7]. The authors performed large-scale genomic analyses of HER2+ breast carcinomas. They identified PIK3CA activation and, for the first time, alterations of the MAP kinase signaling pathway as possible triggers of resistance formation. While the former are present in about one-third of cases, the latter affect about 12.8% of tumors and occur more frequently in the metastatic setting. Also, many refractory carcinomas showed a lack of HER2 amplification. The consequence? The MAP kinase signaling pathway as well as PI3K could serve as additional targets – after all, corresponding agents such as Alpelisib already exist and (like Alpelisib) are partially already approved [1].
Hormone receptor-positive, HER2-negative breast cancer: focus on new agents, subgroup analyses and liquid biopsy
Immediate changes in clinical practice are unlikely to be forthcoming in HR+/HER breast cancer based on the data presented at SABCS, but there were some interesting subgroup analyses and insights into new agents. In addition, the role of liquid biopsy in management was discussed diligently – this could soon provide valuable information on prognosis and treatment response and even influence treatment decisions (Tab. 2) .
At the SABCS, there was news from the pipeline for three active ingredients in particular: Entinostat, samuraciclib and elacestrant. While entinostat, developed in China, is a selective inhibitor of histone deacetylase (HDAC), samuraciclib is a CDK7 inhibitor, and elascestrant is an oral selective estrogen receptordegrader (SERD) [8–10]. While results from phase III trials have already been published for entinostat and elacestrant, samuraciclib is still at an earlier stage of development (Tab. 2) . Nevertheless, its development is remarkable, as it is the first active substance of a new class. This appears to be effective and safe after progression on CDK4/6 inhibitor therapy in a heavily pretreated study population with extremely unfavorable prognosis, according to initial phase I/II results.
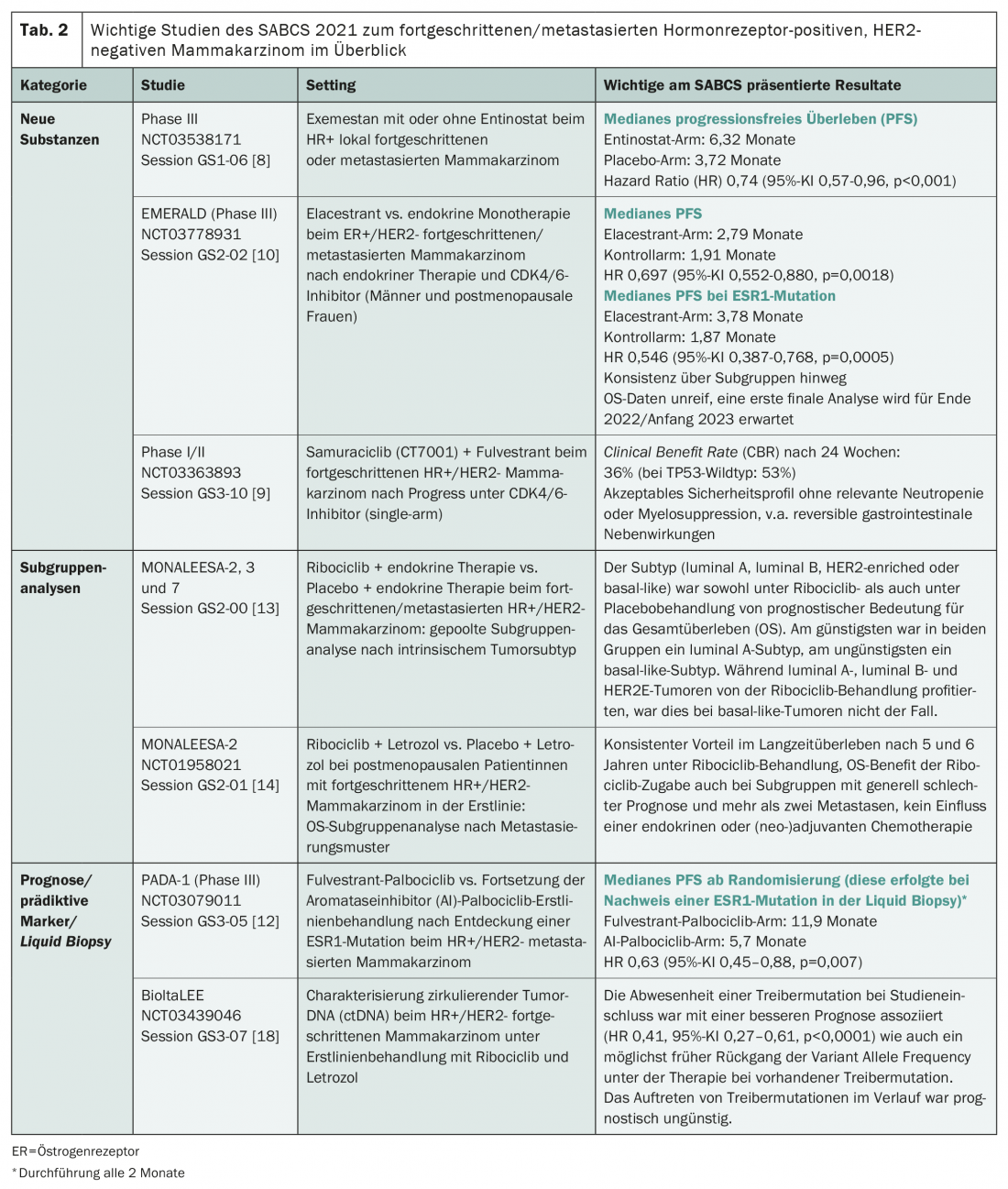
Also after failure of first-line therapy with CDK4/6 inhibitor and endocrine treatment, elacestrant is currently being clinically tested and compared to standard of care – endocrine therapy of the treating physician’s choice: In the Phase III EMERALD study [10]. At the SABCS, initial positive results were presented regarding PFS, with a particularly large effect of treatment in patients with ESR1 mutation. FDA and EMA approvals are still being sought in 2022 [11].
However, ESR1 mutations not only played an important role in the elascestrant study at the symposium, but also in the PADA-1 study [12]. But what exactly are we talking about here? ESR1 mutations, estrogen receptor 1 mutations, are known drivers of resistance to aromatase inhibitors. They are rare in metastatic breast carcinoma, affecting <5% of patients. However, they are more common in progression with first-line treatment with aromatase inhibitors. Under these conditions, the PADA-1 trial addressed the question of whether switching the aromatase inhibitor to fulvestrant would be beneficial if a corresponding genetic alteration was discovered. For this purpose, the blood of affected individuals on first-line palbociclib/aromatase inhibitor therapy was analyzed every two months. If an ESR1 mutation was detected, randomization to the intervention group (palbociclib/fulvestrant) or control group (palbociclib/aromatase inhibitor) was performed. The PFS results presented at SABCS clearly support a switch of therapy in the presence of an ESR1 mutation, with a doubling of median PFS (Table 2) [12].
In addition to detecting ESR1 mutations, liquid biopsy may gain clinical importance in breast cancer in other ways in the coming years. This was shown by a circulating tumor DNA (ctDNA) study [18]. The authors characterized these under first-line treatment with ribociclib and letrozole in terms of their prognostic and predictive value. The absence of a driver mutation at study inclusion was associated with a better prognosis. If a driver mutation was present at baseline, a decline in variant allele frequency as early as possible with therapy proved prognostically favorable. In contrast, the occurrence of driver mutations during the course was shown to be prognostically unfavorable [18]. This study also suggests a potential benefit of liquid biopsy for therapy monitoring and adjustment, if necessary. However, standardized procedures are still lacking at present, and a number of studies will probably have to be carried out before they can be used in everyday clinical practice.
Two subgroup analyses of the MONALEESA studies were also exciting at the SABCS – even if from a familiar background. These successfully investigated the addition of the CDK4/6 inhibitor ribociclib to endocrine therapy in advanced/metastatic HR+ breast cancer in advance – with corresponding approvals from the first line of therapy [1]. Now, both the effects of intrinsic tumor subtype and those of metastatic pattern on overall survival have been examined in more detail (Table 2) [13,14]. The conclusion: the subtype has prognostic relevance under both ribociclib and placebo administration – in contrast to the metastatic pattern. Basal-like and HER2-elevated (HER2E) tumors are generally less prognostic than tumors with luminal subtype. But while HER2E carcinomas respond to the addition of ribociclib, basal-like tumors do not [13]. Based on these data, molecular management in HR+/HER2 breast cancer may become more important in the future. And, the OS benefit with ribociclib persists even with generally poor prognosis; there is a consistent benefit in long-term survival [14].
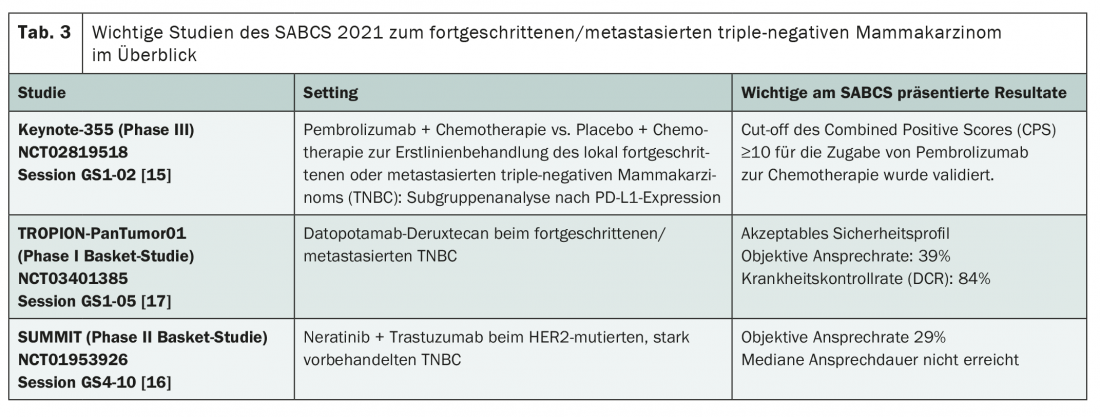
Triple-negative breast carcinoma: an ungrateful adversary
The median OS of metastatic TNBC is now about 14.5 months, and chemotherapy with or without bevacizumab is still considered the first-line standard. Efforts in recent years have produced the first therapeutic approaches in the field of targeted and immunotherapy, but the big breakthrough has not been achieved – and this trend seems to continue on the basis of the studies presented at the SABCS. Developments to date have primarily involved the introduction of PARP inhibitors such as olaparib and talazoparib for BRCA mutation and atezolizumab for PD-L1 expression ≥1%. Also, the antibody-drug conjugate sacituzumab-govitecan has meanwhile been approved from the third line of treatment. Newly, based on the data from the Keynote 355 trial, pembrolizumab could also be added – at least in the case of high PD-L1 expression, this limitation was confirmed at a subgroup analysis presented at SABCS [15]. In addition, the new agent datopotamab-deruxtecan (Dato-DXd) is in the pipeline as well as the combination therapy of trastuzumab and neratinib (Table 3) [16,17]. Both approaches are currently being investigated in basket studies. While the antibody-drug conjugate Dato-DXd, like sacituzumab-govitecan, targets TROP2, neratinib is being tested in HER2-mutated tumors without amplification. The pan-HER TKI may already be used for the extended adjuvant treatment of HR+, HER2-amplified breast carcinoma [1]. Although HER2 mutation is present in only about 2-4% of metastatic breast cancers, it may be worthwhile to test for it in the future – as PD-L1 expression and BRCA mutation already are.
Source: Best of SABCS: https://bestofsabcsnews.com (last accessed 12/16/2021).
Literature:
- Swissmedic drug information: www.swissmedicinfo.ch (last accessed 16.12.2021)
- Cortés J, et al: Trastuzumab deruxtecan (T-DXd) vs trastuzumab emtansine (T-DM1) in patients (Pts) with HER2+ metastatic breast cancer (mBC): Results of the randomized phase III DESTINY-Breast03 study. ESMO Congress 2021, Presidential Symposium 1, Abstract #LBA1.
- Hurvitz S, et al: Trastuzumab deruxtecan (T-DXd; DS-8201a) vs. trastuzumab emtansine (T-DM1) in patients (pts) with HER2+ metastatic breast cancer (mBC): subgroup analyses from the randomized phase 3 study DESTINY-Breast03. GS3-01, SABCS 2021; San Antonio, Texas, USA.
- Kabraji S, et al: Preclinical and clinical efficacy of trastuzumab deruxtecan in breast cancer brain metastases (BCBM). PD4-05, SABCS 2021; San Antonio, Texas, USA.
- Xu B, et al: Updated overall survival (OS) results from the phase 3 PHOEBE trial of pyrotinib versus lapatinib in combination with capecitabine in patients with HER2-positive metastatic breast cancer. GS3-02, SABCS 2021; San Antonio, Texas, USA.
- Xu B, et al: Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021; 22(3): 351-360.
- Ferraro E, et al: Genomic analysis of 733 HER2+ breast cancers identifies recurrent pathways alterations associated with anti-HER2 resistance and new therapeutic vulnerabilities. GS3-03, SABCS 2021; San Antonio, Texas, USA.
- Xu B, et al: A randomized control phase III trial of entinostat, a once weekly, class I selective histone deacetylase inhibitor, in combination with exemestane in patients with hormone receptor positive advanced breast cancer. GS1-06, SABCS 2021; San Antonio, Texas, USA.
- Coombes C, et al: Study of samuraciclib (CT7001), a first-in-class, oral, selective inhibitor of CDK7, in combination with fulvestrant in patients with advanced hormone receptor positive HER2 negative breast cancer (HR+BC). GS3-10, SABCS 2021; San Antonio, Texas, USA.
- Bardia A, et al: Elacestrant, an oral selective estrogen receptor degrader (SERD), vs investigator’s choice of endocrine monotherapy for ER+/HER2- advanced/metastatic breast cancer (mBC) following progression on prior endocrine and CDK4/6 inhibitor therapy: results of EMERALD phase 3 trial. GS2-02, SABCS 2021; San Antonio, Texas, USA.
- Media Release: Menarini Group and Radius Health announce positive phase 3 topline results from the EMERALD trial evaluating elacestrant in breast cancer. 10/20/2021, Radius Health.
- Bidard F-C, et al: Fulvestrant-palbociclib vs continuing aromatase inhibitor-palbociclib upon detection of circulating ESR1 mutation in HR+ HER2- metastatic breast cancer patients: results of PADA-1, a UCBG-GINECO randomized phase 3 trial. GS3-05, SABCS 2021; San Antonio, Texas, USA.
- Carey L, et al: Correlative analysis of overall survival by intrinsic subtype across the MONALEESA-2, -3, and -7 studies of ribociclib + endocrine therapy in patients with HR+/HER2- advanced breast cancer. GS2-00, SABCS 2021; San Antonio, Texas, USA.
- O’Shaughnessy et al: Overall survival subgroup analysis by metastatic site from the phase 3 MONALEESA-2 study of first-line ribociclib + letrozole in postmenopausal patients with advanced HR+/HER2- breast cancer. GS2-01, SABCS 2021; San Antonio, Texas, USA.
- Cortes J, et al: Final results of KEYNOTE-355: Randomized, double-blind, phase 3 study of pembrolizumab + chemotherapy vs placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. GS1-02, SABCS 2021; San Antonio, Texas, USA.
- Jhaveri K, et al: Neratinib + fulvestrant + trastuzumab for hormone receptor-positive, HER2-mutant metastatic breast cancer and neratinib + trastuzumab for triple-negative disease: Latest updates from the SUMMIT trial. GS4-10, SABCS 2021; San Antonio, Texas, USA.
- Krop I, et al: Datopotamab deruxtecan in advanced/metastatic HER2- breast cancer: Results from the phase 1 TROPION-PanTumor01 study. GS1-05, SABCS 2021; San Antonio, Texas, USA.
- Bianchini G, et al: Circulating tumor DNA (ctDNA) dynamics in patients with hormone receptor positive (HR+)/HER2 negative (HER2-) advanced breast cancer (aBC) treated in first line with ribociclib (R) and letrozole (L) in the BioItaLEE trial. GS3-07, SABCS 2021; San Antonio, Texas, USA.
InFo ONCOLOGY & HEMATOLOGY 2022; 10(1): 24-28.