Allergen-specific immunotherapy (AIT) is a causal immunomodulatory therapy. Effective hyposensitization can reduce the risk of impending “floor change” in allergic rhinitis. Current clinical studies confirm that allergy sufferers in children in particular can benefit from this. Both subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT) have been shown to be effective, with SLIT performing particularly well.
Widespread triggers of IgE-mediated immediate-type allergies include tree, grass, and herb pollens [1]. In already sensitized persons, an allergic reaction can be triggered after allergen contact. While symptomatic treatment does not modulate the underlying disease, allergen-specific immunotherapy (AIT) – currently the only causal form of therapy – induces allergen-specific tolerance development by modulating the immune system [1].
Principles of action of allergen-specific immunotherapy (AIT)
The administration of allergen extracts activates specific blocking antibodies, tolerance-inducing cells, and second messengers that prevent further amplification of the immune response triggered by allergens, block the specific immune response, and dampen the inflammatory response in tissues [2]. In sublingual immunotherapy (SLIT), hyposensitization takes the form of tablets that the allergy sufferer usually takes daily. In subcutaneous immunotherapy (SCIT), the allergen is injected under the skin by an allergist. Initially, patients receive only a very small amount of the allergen, which activates certain components of the immune system that prevent the excessive immune response from being inhibited. In the course of further AIT therapy, the amount of allergen is gradually increased until finally the highest dose is reached. As a result, tolerance to the allergen in question can be achieved in the long term, so that the allergic reaction is much weaker when contact occurs again. Overall, AIT should be conducted over at least three years. The efficacy for certain age groups or allergens has been proven in many clinical studies for both subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT) [5]. This applies in particular to allergic rhinitis with or without simultaneous conjunctivitis in pollen and house dust mite allergy as well as allergy to bee and/or wasp venom.
|
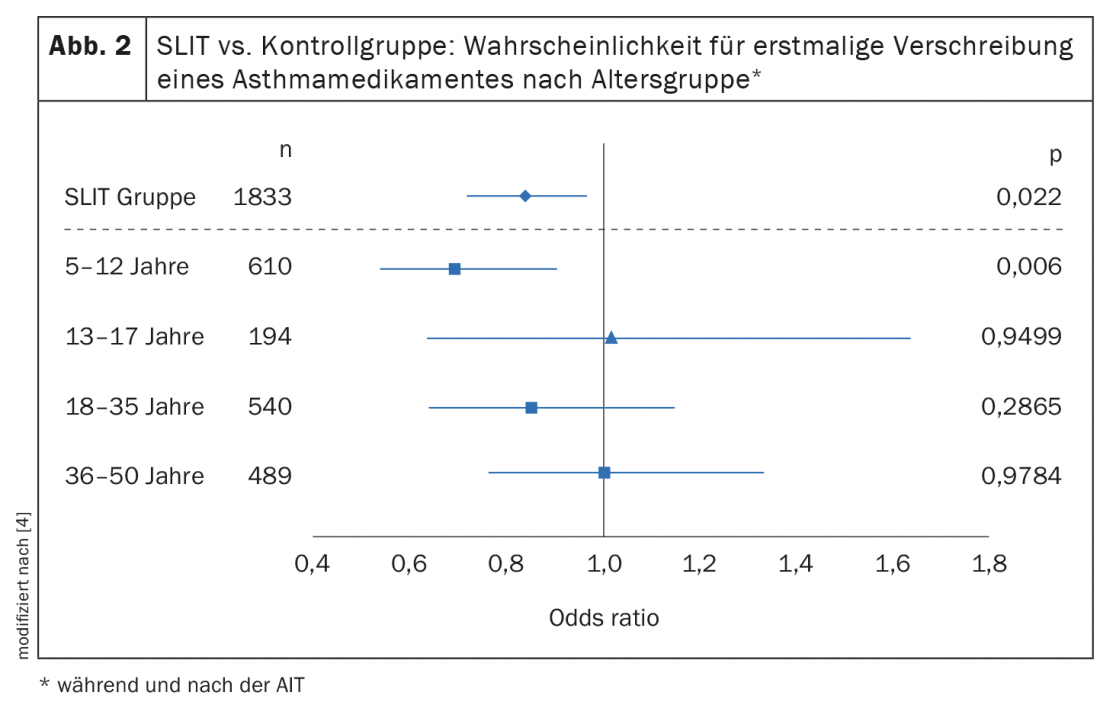
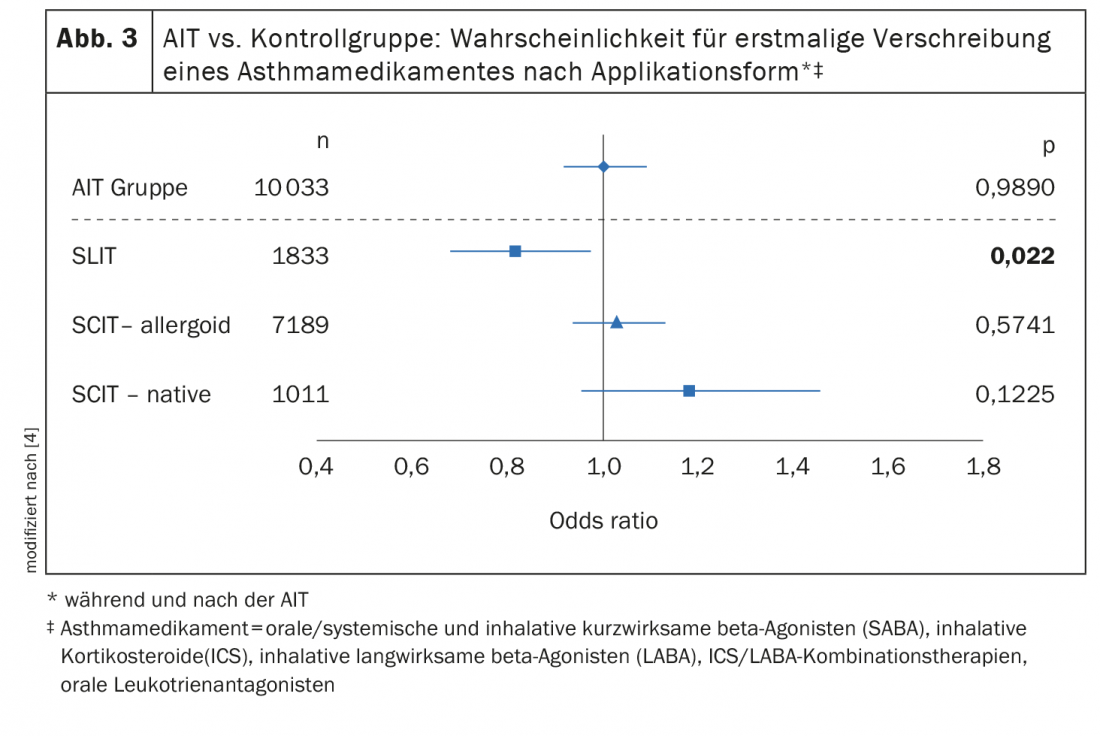
Sublingual immunotherapy (SLIT) achieved the best results In the studies presented on the occasion of the EEACI by Petra Zieglmayer, MD, further showed an influence of the AIT application form and allergy status. Thus, only patients treated with SLIT were found to be significantly less likely to be prescribed a new asthma medication (Fig. 3) [4]. SLIT overall (n=1833), OR: 0.833 (p=0.022); SLIT in 5-12 year olds OR: 0.690 (p=0.006). In addition to age and mode of application, there was evidence that monoallergic patients benefited more than patients with multiple allergies. In summary, the available study data on grass pollen SLIT show a trend toward age-specific effects in reducing the risk of having an indication for an asthma medication during and after SLIT therapy. Children and monoallergic adolescents seem to benefit most from this secondary prevention effect. It might therefore be advisable to start SLIT at an early age and/or at an early stage of the disease. |
Childhood patients in particular benefit from AIT
The noticeable effect of hyposensitization for those affected is an alleviation of symptoms and a reduction in the need for medication. In the long term, it can also prevent disease progression, such as the so-called “floor shift” [1]. In grass pollen allergy, inhalant allergens enter not only the nose but also the bronchial tubes. When the inflammation of the upper airways passes to the lower airways, so that allergic rhinoconjunctivitis develops into allergic asthma, this is also referred to as a “floor change”. The efficacy of sublingual immunotherapy (SLIT) for allergic rhinoconjunctivitis caused by grass pollen has been extensively documented in adults and children [2]. Hyposensitization should be started several months before the pollen season. Aspects of secondary prevention, in particular the reduction of new sensitizations and the reduction of the risk of asthma, are important reasons for the decision to initiate treatment during childhood and adolescence.
SLIT: greatest preventive benefit in children with grass pollen allergy
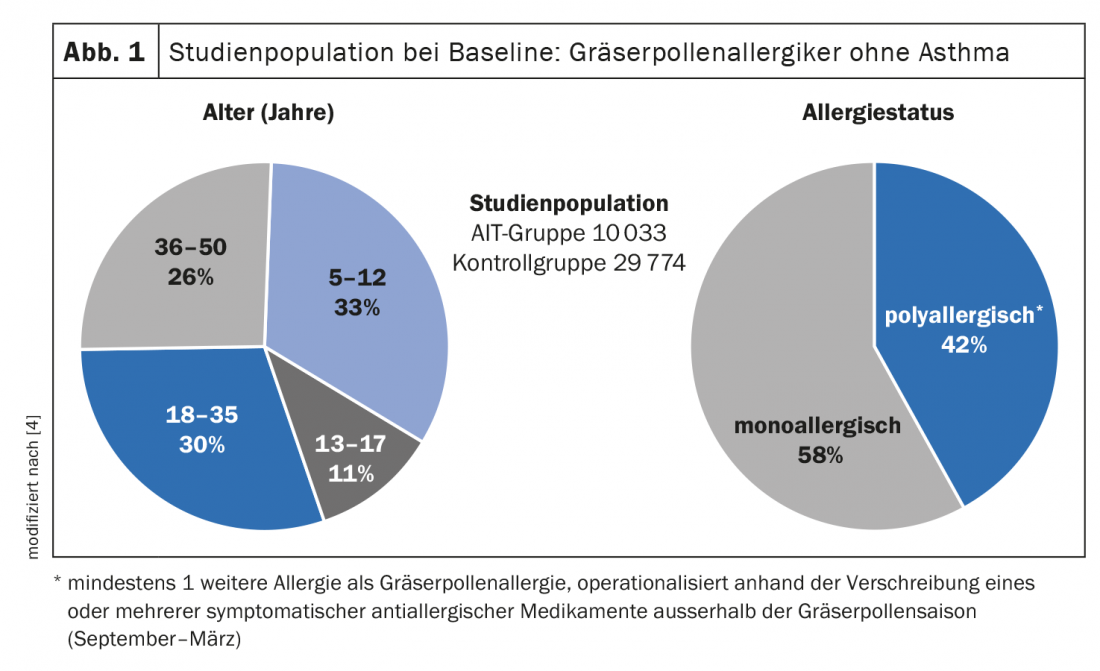
Already in the randomized-controlled double-blinded “Grazax Asthma Prevention” (GAP) study a preventive effect of sublingual immunotherapy with regard to asthma development could be proven in 5-12-year-old children with allergic rhinoconjunctivitis (n=812) [3]. The study period included 3 years of SLIT therapy and 2-year follow-up. At this year’s congress of the European Academy of Allergy and Clinical Immunology (EAACI), further study data were presented indicating that this age group benefits most from preventive effects of AIT. Petra Zieglmayer, MD, Karl Landsteiner Private University of Health Sciences, Krems an der Donau (A), presented the results of a retrospective “real world” study, on the age-specific long-term effects of AIT for grass/cereal pollen [4]. Using the dataset of a German longitudinal prescription database, the age-specific effects of ≥2 years of AIT on the development of bronchial asthma in patients with allergic rhinitis were investigated [4]. Here, an AIT group treated with grass/cereal pollen AIT (including 7 different SLIT/SCIT products) was compared with a control group that received symptomatic treatment only (non-AIT group). Logistic regression analyses compared prescriptions for new asthma medications in both groups as an indicator of asthma incidence. For this purpose, 10 033 and 29 774 patients with allergic rhinitis without asthma in the AIT and non-AIT groups, respectively, were analyzed for age-specific effects. The age distribution (Fig. 1) was as follows [4]: 5-12 years (33%), 13-17 years (11%), 18-35 years (30%), 36-50 years (26%). Across all age groups, 13.4% developed asthma during or after treatment cessation in the AIT group and 14.6% in the non-AIT group. Overall, AIT patients had no lower risk of new asthma medication prescriptions than non-AIT patients (odds ratio [OR]: 1.001, p=0.989). But the age-specific analyses indicate that the younger the patients, the greater the preventive benefit of AIT. For example, patients treated with SLIT showed a significantly reduced risk of new asthma medication prescriptions in the 5-12 year age group (Fig. 2) [4].
Congress: EEACI Annual Meeting
Literature:
- Holzhauser T, et al: Recombinant allergens, peptides, and virus-like particles for allergy immunotherapy [Recombinant allergens, peptides, and virus-like particles for allergy immunotherapy]. Bundesgesundheitsblatt Health Research Health Protection 2020; 63(11): 1412-1423.
- Pfaar O, et al: Guideline on allergen-specific immunotherapy in IgE-mediated allergic diseases: S2k guideline of the German society for allergology and clinical immunology (DGAKI), GPA, AeDA, ÖGAI, SGAI, DDG, DGHNO-KHC, DGKJ, GPP, DGP, BV-HNO, BVKJ, BDP, BVDD. Allergo J Int 2014; 23: 282-319.
- Valovirta E, et al: GAP investigators. Results from the 5-year SQ grass sublingual immunotherapy tablet asthma prevention (GAP) trial in children with grass pollen allergy. J Allergy Clin Immunol 2018; 141(2): 529-538.e13.
- Zieglmayer P: 100048 – Age-specific effects of grass pollen allergen immunotherapy in reducing the risk for new onset asthma: a real world dataset analysis, 01.-03.07.2022.
- Allergy Information Service, www.allergieinformationsdienst.de/therapie/spezifische-immuntherapie/wirksamkeit-und-dauer.html, (last accessed Sept. 06, 2022).
DERMATOLOGIE PRAXIS 2022; 32(5): 52-54