For the treatment of focal epilepsy that cannot be adequately controlled despite previous therapy with at least two anticonvulsants, a new add-on therapy has recently become available for adults – an antiepileptic agent from the carbamate group.
Long-term drug therapy for epilepsy is symptomatic with the aim of reducing the frequency of seizures. Although numerous new antiepileptic drugs have come on the market in recent decades, about 35-40% of newly diagnosed patients do not achieve permanent seizure freedom despite taking several drugs [1,2]. Epilepsy is considered refractory when adequate seizure control is not achieved despite therapy with at least two antiepileptic drugs [3]. In focal seizures, pathological neurological activity occurs in the brain in a circumscribed area of the brain (temporal lobe, frontal lobe, parietal lobe, or occipital lobe) [4]. Clinically, this manifests itself, for example, as rhythmic twitching or cramping of individual limbs, although the affected person is usually conscious.
Since July 2022, cenobamate (Ontozry®) has been approved in Switzerland for adult epileptics as an add-on therapy for focal seizures that are not adequately controlled despite a history of treatment with at least two antiepileptic drugs [5]. According to current knowledge, cenobamate has a dual mechanism of action in that it acts as a sodium channel blocker on the one hand and modulates the presynaptic release of GABA (γ-aminobutyric acid) on the other.
High response rates in clinical trial
The clinical efficacy of cenobamate was evaluated in a randomized-controlled, double-blinded C017 multicenter trial, among others [6]. An inclusion criterion for the C017 study was that at least 3 or 4 partial seizures per 28 days occurred during the 8-week prospective baseline period, with a seizure-free period not to exceed 3 to 4 weeks. This was followed by an 18-week treatment period, including 12 weeks of fixed dose. 397 randomized study participants were included in the modified intention-to-treat maintenance phase population: Cenobamate 100 mg (n=102), Cenobamate 200 mg (n=98), Cenobamate 400 mg (n=95), Placebo (n=102). More than three-quarters of patients were taking two or more antiepileptic drugs concomitantly at the time of enrollment, the most common being levetiracetam, lamotrigine, carbamazepine, and lacosamide [5].
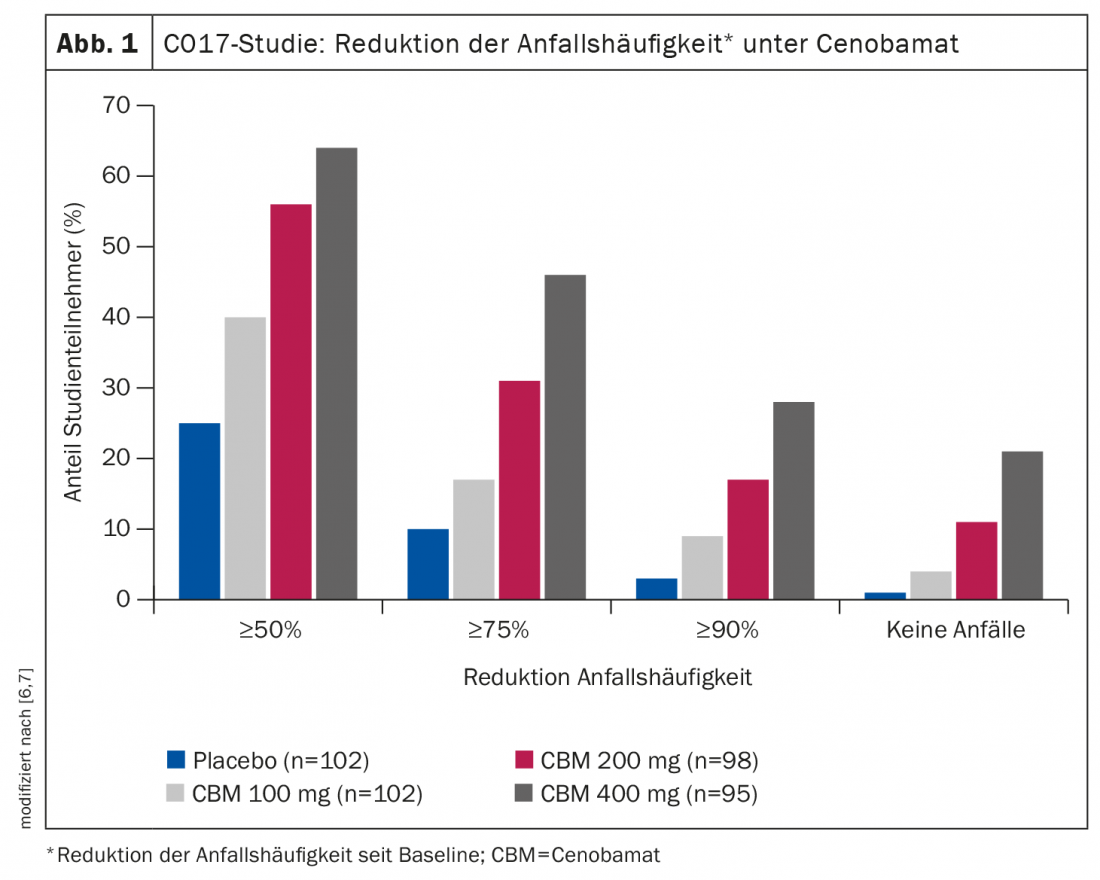
The main study results are shown in Figure 1 [7]: A reduction in seizure frequency of ≥50% from baseline, was achieved by 40% on 100 mg cenobamate, 56% on 200 mg, and 64% on 400 mg versus approximately 25% in the placebo group. Seizure-free rates were 21% in the 400 mg group and 11% in the 200 mg group versus 1% on placebo. Side effects were significantly more frequent with the 400 mg dose. The most common adverse reactions leading to discontinuation were ataxia, dizziness, somnolence, nystagmus, and double vision [8].
Literature:
- Chen Z, et al: Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30-year longitudinal cohort study. JAMA Neurol 2018;75(3): 279-86.
- Tian N, et al: Active epilepsy and seizure control in adults-United States, 2013.
- Institute for Quality and Efficiency in Health Care (IQWiG) 2020, www.iqwig.de/methoden/allgemeine-methoden_version-6-0.pdf?rev=180500, (last accessed 07/22/2022).
- S1 Guideline: First epileptic seizure and epilepsies in adulthood, 2017, https://www.awmf.org/uploads/tx_szleitlinien/030-041l_S1_Erster-epileptischer-Anfall_Epilespien_2018-05.pdf, (last accessed 07/22/2022).
- Swiss Drug Compendium, https://compendium.ch, (last retrieved
- Krauss GL, et al: Safety and efficacy of adjunctive cenobamate (YKP3089) in patients with uncontrolled focal seizures: a multicentre, double-blind, randomised, placebo-controlled, dose-response trial. The Lancet Neurology 2020; 19(1): 38-48.
- Chung SS: Cenobamate: new antiseizure medication with high seizure freedom and dual mechanisms of action. J Pharmacol Pharm Res 2021; 4(2): 2-3, https://researchopenworld.com/wp-content/uploads/2021/07/JPPR-4-424.pdf
- Drugs: Cenobamate: Ontozry®, www.pharmazeutische-zeitung.de/arzneistoffe/daten/2021/cenobamatontozryr152021 (last accessed 07/22/2022).
HAUSARZT PRAXIS 2022; 17(8): 21
InFo NEUROLOGY & PSYCHIATRY 2022; 20(5): 33.