In acute aortic dissection, the aortic wall initially tears from the inside, resulting in separation of the wall layers. The result: an additional lumen is created. What to do?
In acute aortic dissection, there is initially a tear of the aortic wall from the inside and a resulting separation of the wall layers (intima-media of adventitia) through which an additional lumen (which we call the true lumen) is created in addition to the original vessel lumen (which we call the false lumen). The clinical correlate is acute, often migratory chest pain. The classic classification gives a dissection involving the ascending aorta, the definition type A dissection, a dissection involving the aortic arch without the ascending aorta; the definition type non-A-non-B dissection, and a dissection involving the descending aorta, the definition type B dissection [1].
The location of the primary tear (entry) determines the clinical course of acute aortic dissection. The essential principle of the underlying disease is that, after the primary entry has occurred, propagation in the aortic wall occurs with and against the blood flow, leading to widening of the dissection [2].
The location of the primary entry can often be assigned to an aortic segment. However, only its final extension along and against the blood flow determines the clinical course, our definition of the disease (type A, type non-A-non-B or type B) and the resulting immediate or delayed invasive therapeutic measure in addition to drug therapy which initially mostly consists of lowering the arterial blood pressure [3,4].
Propagation against the bloodstream
Acute dissection with a primary entry in the ascending aorta, regardless of propagation and extent, is always referred to as type A dissection, and acute surgical repair is the gold standard because retrograde propagation into the aortic root leads to acute high-grade aortic valve regurgitation due to commissural prolapse and transudation through the aortic wall leads to pericardial tamponade. In this case, aortic rupture is a metachronous process that may occur immediately, with a time delay, or in individual cases not at all [5].
A primary entry in the descending aorta is referred to as a type B dissection, but again, the location of the primary entry and the extent make all the difference for treatment, or whether an acute type B dissection has already caused complications, or complications are expected, or whether it is likely that the dissection will be uncomplicated. We refer to the occurrence of organ malperfusion due to dynamic or static obstruction by the dissecting membrane of lateral or terminal branches of the aorta (spinal cord, gastrointestinal, kidney, and extremities) as “complicated”; however, persistent pain symptoms (pain is a very good biomarker in the course of the first few days) and a difference in blood pressure between the upper and lower extremities (in the sense of pseudocoarctation caused by the dissecting membrane) are also considered “complicated” courses. Any form of bloody pleural effusion and, understandably, any form of covered and uncovered rupture are also considered “complicated.” If all the previously mentioned symptoms are absent, we speak of an “uncomplicated” type B dissection provided that the total diameter of the dissected aorta does not exceed 40 mm.
As “potentially complicated” we consider dissections that fulfill morphological criteria, which often precede the previously mentioned complications, the most important are localization of the primary entry at the inner side of the aortic arch (because in retrograde propagation no anatomical barrier can stop the progression of the process into the aortic arch and into the ascending aorta, which is often prevented at the outer side of the arch by the head and neck vessels) and a short distance between the primary entry and the left subclavian artery (distances less than 2 cm are considered short here) [2,6,7].
Propagation along the bloodstream
For many years, our main focus was almost exclusively on propagation along the blood flow, which is intuitively correct, since a primary entry located immediately at the level of the left subclavian artery shows its effect only in downstream aortic segments, mainly at the level of the visceral and renal vessels, by collapse of the true lumen with malperfusion of the affected end organs. [3,8].
Mechanisms for organ malperfusion
The main cause is static or dynamic compression of the true lumen. The presence or absence of malperfusion is highly dependent on the location of the primary entry and on the dynamics of the dissecting membrane over the cardiac cycle, with the location of the primary entry in angulated aortic segments (such as the aortic arch and proximal descending aorta) showing a stronger correlation with the occurrence of malperfusion [2–5].
Mechanisms for aneurysm formation
The main reason of aneurysm formation is blood pressure difference between true and false lumen and the angle at which blood flow hits the aortic wall. These factors are in turn determined by the localization of the primary entry and also by the number and size of communications between the lumina (formerly also referred to as multiple entries and reentries). Another essential factor is the size of the most distal communication between the lumens, or as the most distal tear (reentry), because – if the reentry is small or absent – the mean arterial blood pressure in the false lumen may be significantly higher than in the true lumen, resulting in an increase in peripheral resistance [9,10].
Active therapeutic strategy
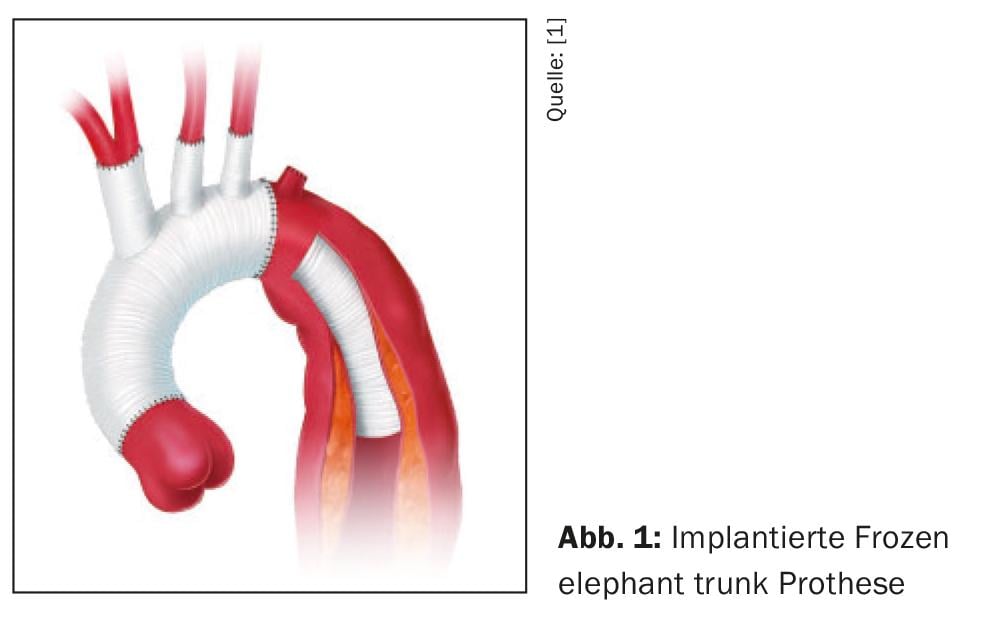
The basic principle of any active therapeutic strategy is always the closure of the primary entry either by resection and vascular replacement in open surgery or by exclusion of the primary entry from the blood stream by insertion of a stent-graft either as “Thoracic endovascular aortic repair (TEVAR)” or in open surgery by the so-called Frozen Elephant Trunk (FET) technique. (Fig. 1). The resulting approximation of the intima-media cylinder and adventitia results in decompression of the true lumen with simultaneous restoration of regular transverse diameters of the downstream aortic segments and thus stabilization of the dissecting membrane In normal cases, this is sufficient for the acute situation, however, the number and size of interluminal communications also have an impact, so that it is always necessary to perform stent-graft lengthening, since large interluminal communications may continue to provoke persistent malperfusion syndromes despite successful closure of the primary entry. Therefore, in individual cases it is also necessary to place non-covered (open) stents over the outlets of the visceral and renal vessels.
Departure of the large lateral branches from the true and/or false lumen
In the course of the acute event, all branches initially continue to depart from the true lumen, and the intima/media cylinders of the large lateral branches initially extend like tubes through the newly formed false lumen to the respective end organ. Since these intima/media cylinders are very fragile, they often rupture sooner or later within the false lumen, which then morphologically results in the circumstance of departure from the false lumen. It is essential to be aware that this is a secondary effect and that the resulting communication between the lumina is always the size of the exit diameter of the side branch. For this reason, blood flow via this communication between true and false lumen is sufficient for end-organ perfusion in most cases, and occlusion of the primary entry with suppression of perfusion in the false lumen has no detrimental effect on blood flow to the respective end organs regardless of whether open surgery, TEVAR, or the FET technique has been used. [12].
Summary of pathophysiology
One should always be aware that the currently used definitions (type A, type non-A-non-B, type B dissection) are only approximations to describe the same pathophysiological process in which the beginning (primary entry) and the respective end (along and against the blood flow) of the dissection make the fundamental difference in the clinical course and the need for an active treatment strategy.
What is the best treatment strategy for uncomplicated type B dissection?
For many years, acute type B dissection was interpreted as the “harmless” aortic dissection in its clinical course (especially in comparison with type A dissection, which often progresses dramatically), because action was rarely required in the acute situation [13]. However, a closer look into the natural history of the disease has shown that a large number of patients develop complications at given time points and then require active treatment [14]. Causes and timing have been found to vary with organ malperfusion, retrograde propagation into the aortic arch and ascending aorta (retrograde type A dissection) being prominent in the acute phase of the disease and then aneurysm formation predominating in the subacute and chronic phases [15]. The answer to the question of which patients will follow which primary course and who will develop secondary complications has been demonstrated primarily in morphologic and functional parameters and has led to the definition of high-risk groups [16,17].
Timing of the disease
The acute phase is defined from the immediate onset of the primary pain event to day 14 thereafter, between day 15 and day 90 we refer to it as the subacute phase, and from day 91 we refer to it as the chronic phase [13].
INSTEAD trial
The INSTEAD trial (Investigation of STEnt Grafts in Aortic Dissection) and long-term follow-up (INSTEAD-XL trial) prospectively compared whether additional prophylactic stent-graft implantation (TEVAR) has a benefit compared with optimal drug therapy alone in patients with uncomplicated type B dissection between 2 and 52 weeks after the acute event. After 5 years, a significantly positive effect was shown in patients receiving additional prophylactic TEVAR in terms of aortic-related survival and on progression of the underlying disease [18,19].
ADSORB trial
The ADSORB Trial (Acute Dissection Stent Grafting or Best Medical Treatment) prospectively compared whether additional TEVAR has a benefit compared with optimal medical therapy alone in patients with acute uncomplicated type B dissection between 0 and 2 weeks after the acute event. The primary end point here was a combination of thrombosis status of the true lumen and, aortic dilatation or adverse aortic events (rupture) at one year. The conclusion drawn was that TEVAR could achieve both positive aortic remodeling and diameter reduction with consecutive (intentional) thrombosis of the false lumen, but long-term results had to be awaited, which are not available at this time [20].
These two studies represent the highest level of evidence available at this time. The INSTEAD XL Trial in particular has led to a modification of the latest ESC Aortic Guidelines and has prophylactically elevated TEVAR in uncomplicated type B dissection to a Class IIA, Level of Evidence B recommendation.
Natural history of uncomplicated type B dissection
After the acute phase, chronification occurs, with the dissecting membrane undergoing a transitional phase between the highly elastic and fragile acute state to a static state. The effect of complete reapposition of wall layers is best in the first few weeks after the acute event [21]. It is very rare for complications such as organ malperfusion or retrograde propagation into the ascending aorta (retrograde type A dissection) to still occur at this stage; however, there is always an increase in the maximum transverse diameter of the aorta. This circumstance of diameter increase can be anticipated by morphological criteria or traced by computed tomography angiography (CTA) controls and can then be stopped by TEVAR at an early stage and in the best case reversible [16].
Optimal treatment strategy for acute uncomplicated type B dissection.
Based on the currently available evidence, it is correct to recommend optimal drug therapy as the basis of treatment for any acute type B dissection. Accurate morphologic analysis allows identification of “high-risk” subgroups that benefit prognostically from early TEVAR. This is ultimately a very large number of patients, up to 80% in some collectives. The INSTEAD Trial and its 5-year follow-up (INSTEAD XL Trial) provide us with the best currently available evidence for the benefit of an endovascular strategy in patients with uncomplicated type B dissection. Prophylactic TEVAR should be considered if the anatomy is suitable [19]. In recent years, this expanded knowledge has already found its way into international guidelines and expert consensus documents [22,23].
Case study 1 – Acute complicated type B dissectionA 57-year-old man suffers sudden acute chest pain that begins between the shoulder blades and radiates migratory to the groin. After 5 minutes, he first develops sensory disturbances and then paraparesis and finally paraplegia of the right lower extremity. The emergency physician called to the scene clinically detects a pulse deficit in the right groin and suspects an acute aortic dissection in view of the wandering pain symptoms and the pulse deficit. In addition, the patient clinically develops an acute abdomen with pain and guarding. He is transferred to the nearest emergency department where a CTA of the entire aorta is performed based on the suspected diagnosis. Results of CTA: CTA shows a type B dissection with the primary entry located at the inner aortic arch essentially without spacing from the left subclavian artery. There is collapse of the true lumen at the level of the visceral and renal vessels, and the right common iliac artery is also functionally occluded. Clinical course: Serum lactate increases to 10 mmol/l and diuresis stops. Acute action is required to close the primary entry to decompress the true lumen and restore blood flow to the downstream aortic segments and right lower extremity. Constellation favoring a primary TEVAR. As part of the diagnosis of CTA, the head and neck vessels were also examined; the left vertebral artery is small and does not contribute to the competence of the circle of Willisi. In this constellation, primary TEVAR with overstenting of the left subclavian artery outlet can be performed to resolve the acute situation, the likelihood of triggering acute upper extremity ischemia is low, but peripheral oxygen saturation measurement of the left arm should be performed and the further procedure should additionally depend on the clinical course (exertional claudication). Constellation favoring TEVAR and simultaneous revascularization of the left subclavian artery: The left vertebral artery is a very large vessel and the circle of Willisi is interrupted, thus- in the case of hypoplastic right vertebral artery, the left is of fundamental importance. Therefore, carotid-subclavian bypass is performed before stent-graft implantation to preserve posterior cerebral circulation and to maintain inflow into the anterior spinal artery (avoiding hindmarch ischemia). Constellation favoring primary FET implantation: The patient has an ascending aorta and an aortic arch aneurysm with a maximum transverse diameter of 5 cm, which does not provide an adequate proximal landing zone and prevents TEVAR from being performed. |
“High-risk” subgroups with acute type B dissection- localization of primary entry.
The location of the primary entry at the inner side of the aortic arch has been found to be one of the most significant morphologic risk factors for the preexistence or for the occurrence of complications of acute type B dissection; this is especially true for the occurrence of organ malperfusion and for retrograde type A dissections within the first 14 days after the acute event [2,16,17].
Distance between the primary entry and the exit of the left subclavian artery
The distance between the primary entry and the exit of the left subclavian artery is also a significant predictor with the risk decreasing significantly with greater distance [16, 24-26].
Size of the primary entry
The size of the primary entry is also prognostically relevant, especially with regard to early increase in the maximum transverse diameter of the aorta; above 10 mm maximum transverse diameter, the risk increases significantly for early aortic expansion and rupture. The best method, but rarely used primarily for pragmatic reasons, is transesophageal echocardiography (TEE) [25]. The reason for this is also due to a blood pressure spike often associated with TEE, which one would generally want to avoid in aortic dissection.
Total diameter of the aorta and diameter of the false lumen
An overall diameter of 40 mm or greater initially measured at diagnosis of CTA and an initial diameter of the fibrous lumen equal to or greater than 22 mm have also been shown to be predictors of size increase over time and warrant endovascular stent-graft placement [17,27,28].
Number and size of communications between lumina
The number and size of communications between lumina throughout the thoracoabdominal aorta have also been shown to be major determinants of size increase over time. Even after TEVAR, large-caliber communications between lumens distal to the stent graft may functionally act like a new primary entry. A similar mechanism can be observed when, after TEVAR, the dissection membrane at the distal stent-graft end tears inward into the false lumen due to excessive shear forces (usually with oversized stent-graft), then this is referred to as distal Stent-graft Induced New Entry-(dSINE) [29,30].
Thrombosis of the false lumen to varying degrees
Partial thrombosis of the false lumen has also been shown to be a prognostic surrogate for adverse outcome. Partial thrombosis can often be characterized as a flow phenomenon with more modern imaging, but the hypothesis of increased mean arterial blood pressure in the false lumen due to delayed outflow (small distal interluminal communication) remains the same because peripheral resistance is increased in the false lumen [10].
TEVAR for every patient with uncomplicated type B dissection?
The purpose of prophylactic TEVAR remains to avoid later aneurysm formation and associated late complications, since malperfusion and spontaneous retrograde type A dissection occur only during the acute phase. However, retrograde type A dissection can also occur in the setting of TEVAR, and this circumstance, in combination with other very rare but occurring procedure-related complications such as stroke and symptomatic spinal cord ischemia, are the reasons why the indication must continue to be well justified. An ectatic ascending aorta, an ectatic aortic arch, and extensive transposition procedures to achieve an adequate proximal landing zone are additional risk factors [31,32].
Case study 2 – Acute uncomplicated type B dissectionA 64-year-old woman experiences acute chest pain that begins between the shoulder blades and continues to diaphragm level. She loses consciousness for 30 seconds, then the pain gets better. The emergency physician makes a suspected clinical diagnosis of acute coronary syndrome and refers the patient to the nearest emergency department with a catheter station for further diagnosis. Further examinations: A blood draw is performed, cardiac enzymes are normal, but D-dimer is elevated. CTA is performed to rule out pulmonary embolism or aortic dissection. CTA shows a type B dissection with a primary entry at the aortic arch exterior without evidence of malperfusion. constellation that favors a primarily conservative approach: The distance of the primary entry to the left subclavian artery is 3 cm, the maximum transverse diameter of the primary entry is 5 mm, the total diameter of the aorta is 37 mm, and the diameter of the false lumen is 16 mm. There are no imaging or clinical signs of malperfusion. Thus, a low-risk situation exists in this constellation and a primarily conservative approach with optimal drug therapy and control CTA are initiated. Constellation favoring prophylactic TEVAR:
These two constellations may be considered “high-risk” despite formal “uncomplicated” type B dissection, and early TEVAR therapy to close the primary entry should be performed. Basically – if TEVAR is performed under planned circumstances, a CSF drain should be placed prior to the procedure for spinal cord protection (the CSF drain is the fasciotomy of the spinal cord). |
Risk assessment of potential complications associated with therapy
The residual risk of stroke is in the low single-digit percentage range. In this case, an accurate statement can be made in advance by assessing the diagnostic CTA with regard to arteriosclerotic lesions near the outlets of the supraaortic branches and thus comprehending or ruling out a potential embolic risk by catheter manipulation.
A major issue remains symptomatic spinal cord ischemia of varying degrees, ranging from sensory disturbances to complete paraplegia. Here, a quantum leap in knowledge of the underlying mechanisms and their prevention has occurred in recent years. In this regard, it should be noted that the spinal cord, analogous to Riolan’s arcade of visceral arterial supply, has extra- and intraspinal back-up mechanisms that can compensate for acute and chronic inadequate perfusion to some extent. First, the “four-territory” concept will be discussed here [33]. This presupposes the anatomical existence of four arterial tributaries of the spinal cord, which are equal in value, namely the subclavian artery (with its continuation into the vertebral artery and thus into the anterior spinal artery, the thoracic segmental arteries (intercostal vessels), the lumbar segmental arteries (lumbar vessels) and the internal iliac artery, all of which form tributaries to the anterior radiculomedullary arteries and thus to the anterior spinal artery. If occlusion of one of these arterial tributaries occurs (as an example, covering multiple thoracic segmental arteries with a stent-graft), this does not significantly affect an increased risk of symptomatic spinal cord ischemia. However, if two tributaries are occluded at the same time (e.g., additional overstenting of the left subclavian artery), this significantly increases the risk of paraplegia because this extent of reduced blood flow can no longer be compensated. Even a single episode of marked hypotension may already increase the risk of paraplegia (e.g., acute TEVAR in rupture). Nature has the ability to compensate for acute inadequate blood supply to, for example, the thoracic section of the spinal cord via the autochthonous back muscles and to induce sprouting of capillary vessels in the inadequately supplied area [34]. Within the spinal cord there are even -preformed collateral vessels that can step in in such situations, but the capacity of this collateral network is just limited and any form of intra- or postoperative hypotension contributes to the risk [35].
An extremely helpful measure in the elective situation for the prevention of symptomatic spinal cord ischemia is the application of a cerebrospinal fluid drainage before surgery. Every tissue, including the spinal cord, reacts to acute ischemia with tissue swelling, but the spinal cord is very limited in its ability to swell in the narrow spinal canal due to the presence of its carrier fluid, i.e. the cerebrospinal fluid, and – analogous to limb ischemia without fasciotomy – the capillary bed is squeezed and a vicious circle ensues, which then ends with organ loss; in this respect, the cerebrospinal fluid drainage can be described as a fasciotomy of the spinal cord [36]. Even in cases of secondary symptomatic spinal cord ischemia, e.g., a few days after surgery, the application of a CSF drainage is often associated with impressive clinical success.
Another very useful monitoring measure in TEVAR is the measurement of motor and somatosensory evoked potentials (MEPs/ SSEPs) [37]. This makes it possible to detect ischemic areas functionally at an early stage and to counteract them accordingly, e.g. by increasing the mean arterial blood pressure or by raising the hematocrit; these measures can also be very helpful in the early phase of acute spinal cord ischemia.
In recent years, the FET technique has been established in the treatment of a variety of acute and chronic thoracic aortic pathologies but also in patients with complex type B dissection but without an adequate proximal landing zone for stent-graft anchorage. Although this indication is, rare, it should be considered if the assessed risk for retrograde type A dissection is high. The results of this procedure in this indication are very good in experienced centers [33]. Classical open surgery via left lateral thoracotomy, on the other hand, is no longer of any importance today.
Open fenestration surgery, in which the dissecting membrane is partially excised, is also obsolete today. Furthermore, the sole interventional treatment of a malperfused end organ has no value, because the cause of malperfusion would still exist due to the open primary entry and would remain unresolved.
Choosing the right time for treatment
Currently available evidence suggests that, assuming clinical stability, a longer interval between acute event and TEVAR (greater than 2 weeks) is favorable in terms of procedure-related complications [38,39].
Recommended diagnostic and treatment algorithm
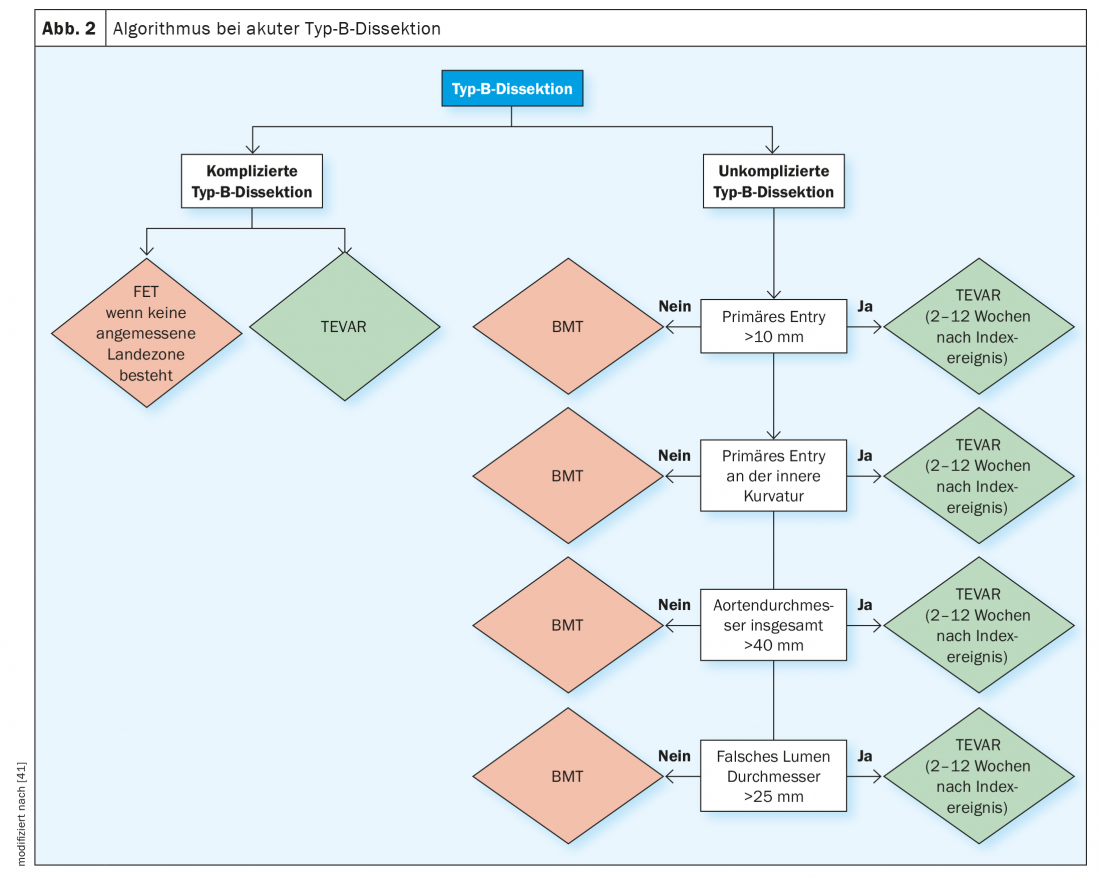
The diagnosis of acute and subacute uncomplicated type B dissection requires a complex evaluation algorithm in which the surrogate parameters for early and late complications should be evaluated before the diagnosis of “uncomplicated” type B dissection is made. Figure 2 shows the diagnostic algorithm that is also recommended by the major professional societies to define these high-risk subgroups and then to follow the appropriate treatment path.
Take-Home Messages
- The incidence of truly uncomplicated type B dissection is lower than previously thought.
- A stepwise diagnosis of exclusion to confirm or rule out “high-risk” surrogates should be applied using CT angiography before establishing the diagnosis of “uncomplicated.”
- The potential of the aorta for positive remodeling (diameter decrease, approximation of the wall layers) decreases with time, this should be taken into account when choosing the time of treatment (“window of opportunity of 90 days”).
- Attention to maintaining spinal cord perfusion via the four major arterial tributaries to the greatest extent possible is a critical factor in reducing the residual risk of symptomatic spinal cord ischemia.
- CSF drainage is a highly effective measure for the prevention and also the therapy of symptomatic spinal cord ischemia.
- In patients who require treatment but are at high risk for retrograde type A dissection, the FET technique should be considered.
Literature:
- Czerny M, Schmidli J, Adler S, et al: Current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch- an expert consensus document of the European Association for Cardio-Thoracic Surgery (EACTS) and the European Society of Vascular Surgery (ESVS). Eur J Cardiothorac Surg 2019(55): 133-162.
- Loewe C, Czerny M, Sodeck GH, et al: A new mechanism by which an acute type B aortic dissection is primarily complicated, becomes complicated, or remains uncomplicated. Ann Thorac Surg 2012; 93: 1215-1522.
- Ante M, Mylonas S, Skrypnik D, et al: Prevalence of the computed tomographic morphological DISSECT predictors in uncomplicated Stanford type B aortic dissection. Eur J Vasc Endovasc Surg 2018; 56: 525-533.
- Weiss G, Wolner I, Folkmann S, et al: The location of the primary entry tear in acute type B aortic dissection affects early outcome. Eur J Cardiothorac Surg 2012; 42: 571-576.
- Erbel R, Aboyans V, Boileau C, et al: 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases- Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult- The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014; 35: 2873-2926.
- Rylski B, Reser D, Kari F, et al: Acute non A, non B aortic dissection: definition, treatment and outcome. Eur J Cardiothorac Surg 2017; 52: 1111-1117.
- Grimm M, Loewe C, Gottardi R, et al: Novel insights into the mechanisms and treatment of intramural hematoma affecting the entire thoracic aorta. Ann Thorac Surg 2008; 86: 453-456.
- Trimarchi S, Jonker FH, van Bogerijen GH, et al: Predicting aortic enlargement in type B aortic dissection. Ann Cardiothorac Surg 2014; 3: 285-291.
- Czerny M, Eggebrecht H, Rousseau H, et al: Distal stent-graft induced new entry after TEVAR or FET – insights into a new disease from EuREC. Ann Thorac Surg 2020, e-pub ahead of print.
- Tsai TT, Evangelista A, Nienaber CA, et al: International Registry of Acute Aortic Dissection. Partial thrombosis of the false lumen in patients with acute type B aortic dissection. N Engl J Med 2007; 357: 349-59.
- Evangelista A, Isselbacher EM, Bossone E, et al: Insights from the International Registry of Acute Aortic Dissection: A 20 year experience of collaborative clinical research. Circulation 2018; 137: 1846-1860.
- Czerny M, Rodler S, Fakhimi S, et al: Mid-term results of TEVAR in patients with aneurysms involving the descending aorta originating from chronic type B dissections. Ann Thorac Surg 2010; 90:90-94.
- Evangelista A, Isselbacher EM, Bossone E, et al: Insights from the International Registry of Acute Aortic Dissection: A 20 year experience of collaborative clinical research. Circulation 2018; 137: 1846-1860.
- Pape LA, Awais M, Woznicki EM, et al: Presentation, diagnosis, and outcomes of acute aortic dissection: 17-year trends from the international registry of acute aortic dissection. J Am Coll Cardiol 2015; 66: 350-358.
- Zeeshan A, Woo EY, Bavaria JE, et al: Thoracic endovascular aortic repair for acute complicated type B aortic dissection: superiority relative to conventional open surgical and medical therapy. J Thorac Cardiovasc Surg 2010; 140(6 Suppl): S109-15.
- Trimarchi S, Jonker FH, van Bogerijen GH, et al: Predicting aortic enlargement in type B aortic dissection. Ann Cardiothorac Surg 2014; 3: 285-291.
- Ante M, Mylonas S, Skrypnik, et al: Prevalence of the computed tomographic morphological DISSECT predictors in uncomplicated Stanford type B aortic dissection. Eur J Vasc Endovasc Surg 2018; 56: 525-533.
- Nienaber C, Rousseau H, Eggebrecht H, et al: A randomized comparison of strategies for noncomplicated type B aortic dissection – the INvestigation of STEnt-grafts in Aortic Dissection (INSTEAD) trial. Circulation 2009; 120: 2519-2528.
- Nienaber CA, Kische S, Rousseau H, et al: Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Intervent 2013; 6: 407-416.
- Brunkwall J, Kasprzak P, Verhoeven E, et al: Endovascular repair of acute uncomplicated aortic type B dissection promotes aortic remodeling ; 1 year results of the ADSORB trial. Eur J Vasc Endovasc Surg 2014; 48: 285-291. erratum in Eur J Vasc Endovasc Surg 2015; 50: 130.
- Moulakakis KG, Mylonas SN, Dalainas I, et al: Management of complicated and uncomplicated acute type B dissection. A systematic review and meta-analysis. Ann Cardiothorac Surg 2014; 3: 234-246.
- Erbel R, Aboyans V, Boileau C, et al: 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases- Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult- The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014; 35: 2873-2926.
- Grabenwöger M, Alfonso F, Bachet J, et al: ESC/EACTS position statement on thoracic endovascular aortic repair (TEVAR). Eur J Cardiothorac Surg 2012; 42: 17-24.
- Weiss G, Wolner I, Folkmann S, et al: The location of the primary entry tear in acute type B aortic dissection affects early outcome. Eur J Cardiothorac Surg 2012; 42: 571-576.
- Evangelista A, Salas A, Ribera A, et al: Long-term outcome of aortic dissection with patent false lumen: predictive role of entry tear size and location. Circulation 2012; 125: 3133-3141.
- Codner JA, Lou X, Duwayri YM, et al: The distance of the primary intimal tear from the left subclavian artery predicts aortic growth in uncomplicated type B aortic dissection. J Vasc Surg 2019; 69: 692-700.
- Song JM, Kim SD, Kim JH, et al: Long-term predictors of descending aortic aneurysmal change in patients with aortic dissection. J Am Coll Cardiol 2007; 50: 799-804.
- Fattori R Cao P, De Rango P, et al: Interdisciplinary expert consensus document on endovascular treatment of type B aortic dissection. J Am Coll Cardiol 2013; 61: 1661-1678.
- Czerny M, Eggebrecht H, Rousseau H, et al: Distal stent-graft induced new entry after TEVAR or FET – insights into a new disease from EuREC. Ann Thorac Surg 2020, in press.
- Lou X, Duwayri YM, Jordan WD Jr, et al: The Safety and Efficacy of Extended TEVAR in Acute Type B Aortic Dissection. Ann Thorac Surg 2020, in press.
- Cochennec F, Tresson P, Cross J, et al: Hybrid repair of aortic arch dissections. J Vasc Surg 2013; 57: 1560-1567.
- Eggebrecht H, Thompson M, Rousseau H, et al; on behalf of the European Registry on Endovascular Aortic Repair Complications. Retrograde ascending aortic dissection during or after thoracic aortic stent-graft placement-insights from the European Registry on endovascular aortic repair complications (EuREC). Circulation 2009; 120: S276-281.
- Czerny M, Eggebrecht H, Sodeck G, et al: Mechanisms of symptomatic spinal cord ischemia after TEVAR- Insights from the European Registry of Endovascular Aortic Repair Complications (EuREC). J Endovasc Ther 2012; 19: 37-43.
- Etz CD, Kari FA, Mueller CS, et al: The collateral network concept: remodeling of the arterial collateral network after experimental segmental artery sacrifice. J Thorac Cardiovasc Surg 2011; 141: 1029-1036.
- Heber U, Mayrhofer M, Gottardi R, et al: The intraspinal arterial collateral network – a new anatomical basis for understanding and preventing paraplegia during aortic repair. Eur J Cardiothorac Surg 2020, in press.
- Etz C, Weigang E, Hartert M, et al: Contemporary spinal cord protection during thoracic and thoracoabdominal aortic surgery and endovascular aortic repair: A position paper of the vascular domain of the European Association for Cardio-Thoracic Surgery. Eur J Cardiothorac Surg 2015; 47: 943-957.
- Maier S, Shcherbakova M, Beyersdorf F, et al: Benefits and risks of prophylactic cerebro-spinal fluid catheter and evoked potential monitoring in symptomatic spinal cord ischemia low-risk thoracic endovascular aortic repair. Thorac Cardiovasc Surg 2018; 67: 379-384.
- Kreibich M, Berger T, Morlock J, et al: The frozen elephant trunk technique for the treatment of acute complicated type B aortic dissection. Eur J Cardiothorac Surg 2018; 53: 525-530.
- Desai ND, Gottret JP, Szeto WY, et al: Impact of timing on major complications after thoracic endovascular aortic repair for acute type B aortic dissection. J Thorac Cardiovasc Surg 2015; 149(2 Suppl): S151-156.
- Heijmen R, Fattori R, Thompson M, et al: Mid-term outcomes and aortic remodeling after thoracic endovascular repair for acute, subacute, and chronic aortic dissection: the VIRTUE Registry. Eur J Vasc Endovasc Surg 2014; 48: 363-371.
- Czerny M, Pacini D, Aboyans V, et al.: Current options and recommendations for the use of thoracic endovascular aortic repair in acute and chronic thoracic aortic disease: an expert consensus document of the European Society for Cardiology (ESC) Working Group of Cardiovascular Surgery, the ESC Working Group on Aorta and Peripheral Vascular Diseases, the European Association of Percutaneous Cardiovascular Interventions (EAPCI) of the ESC and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2021 Jan 4;59(1): 65-73.
CARDIOVASC 2022; 21(3): 6-12